40 energy level diagram for helium
Apr 19, 2011 · Today let us discuss the working of helium neon laser: Pumping of He atoms: When electric discharge is passed through the gas mixture of He and Ne, electrons are accelerated down the discharge tube in which mixture of He-Ne is placed. These accelerated electrons collide with helium atoms and excite them to higher energy levels (let us say F2 ...
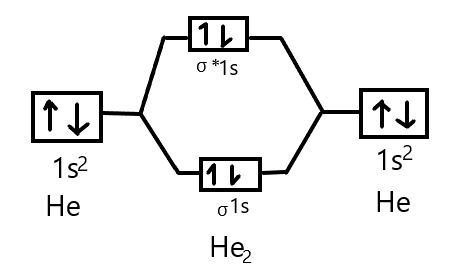
Molecular Orbital Energy Level Diagrams: Hydrogen and Helium "Count and Sort" Algorithm for Building Molecular Orbital Energy Level Diagrams . 1. Write the electron configuration for each atom , and count the total number of valence electrons. If you're working with an ion, adjust the valence electron count according to the charge. 2.
After the first energy level contains 2 electrons (helium), the next electrons go into the second energy level. After the second energy level has 8 electrons (neon), the next electrons go into the third energy level. After the third energy level has 8 electrons (argon), the next 2 electrons go into the fourth energy level.
Energy level diagram for helium
Energy level diagram for helium. Excited-state decays to the ground state are indicated, along with laser transitions from the metastable 2 S 1 3 state. Reuse & Permissions
Helium Neon Laser Energy Level Diagram Here upward transition shows the absorption of energy from the pumping source by Helium atom. While down ward transition shows the emission of energy / light or lasing present in the Neon atom only. In diagram above there are 3 down word energy transitions for Neon that produce lasing.
ground state and the two lowest excited states of helium. To put these results into context, please look at the energy level diagram in Section 5.2.1 of Gri ths. This truncated-matrix approach to the helium atom, including the Mathematica code that I’ll show in class, is based on a recent article by Robert C. Mass e and
Energy level diagram for helium.
He - Ne LASER || PRINCIPLE, CONSTRUCTION, WORKING, ENERGY LEVEL DIAGRAM OF HELIUM NEON LASERABOUT THIS VIDEOA helium-neon laser or He-Ne laser, is a type of ...
Download scientific diagram | Energy-level diagram of pertinent helium singlet states involved in observed photons and important cascade routes. from publication: Electron-impact excitation of the ...
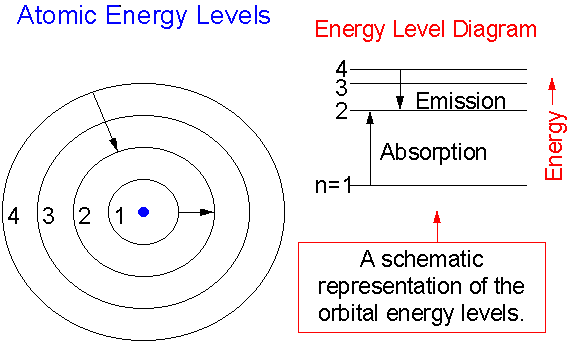
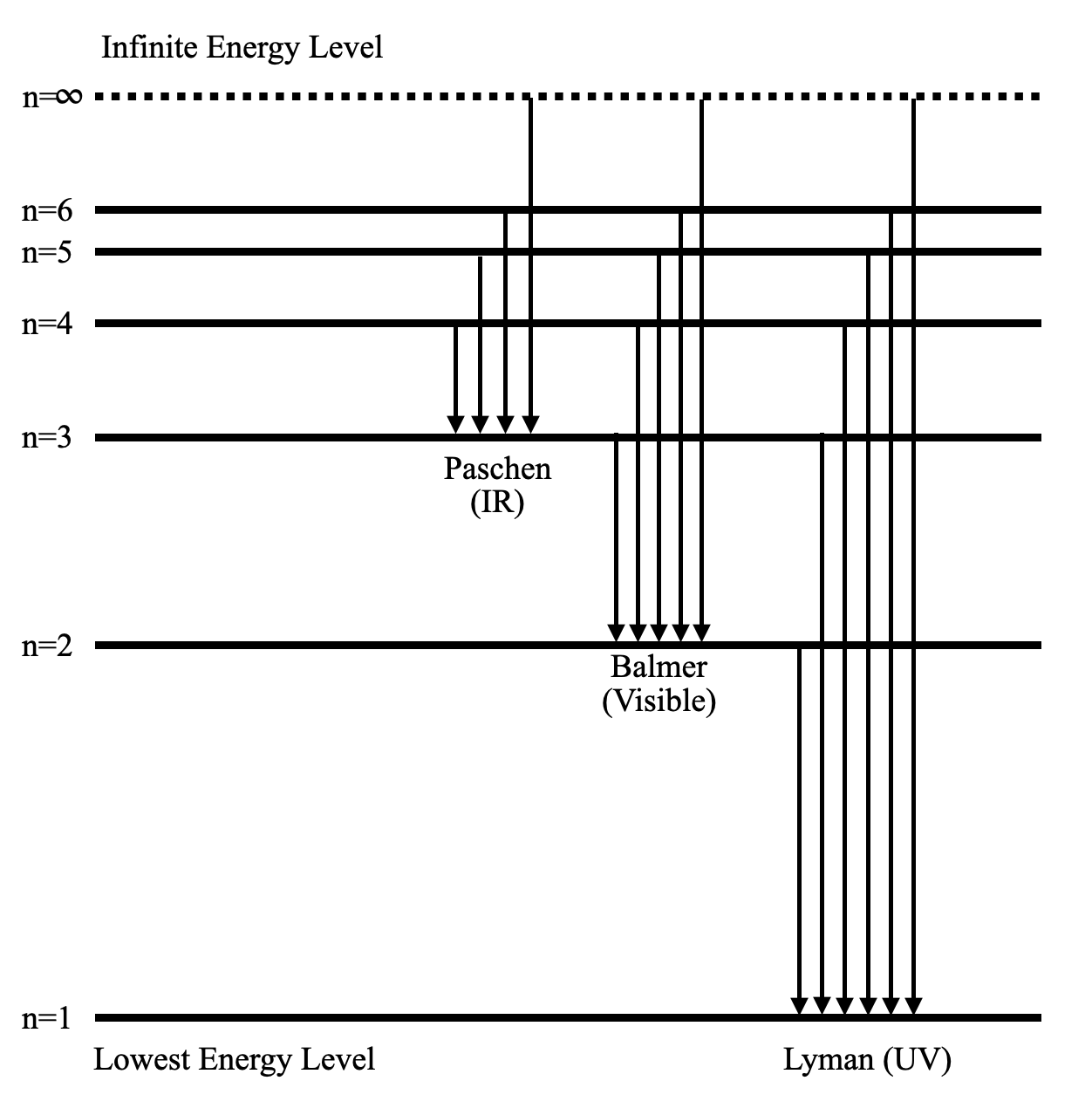
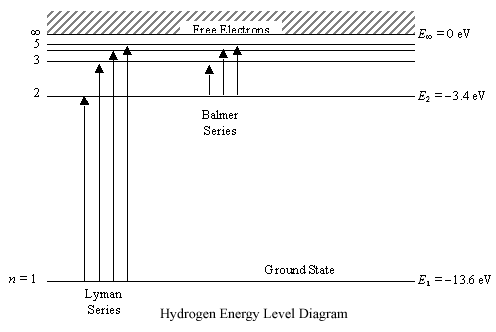
PhysicsLAB: Energy-Level Diagrams. Energy level diagrams are a means of analyzing the energies electrons can accept and release as they transition from one accepted orbital to another. These energies differences correspond to the wavelengths of light in the discreet spectral lines emitted by an atom as it goes through de-excitation or by the ...
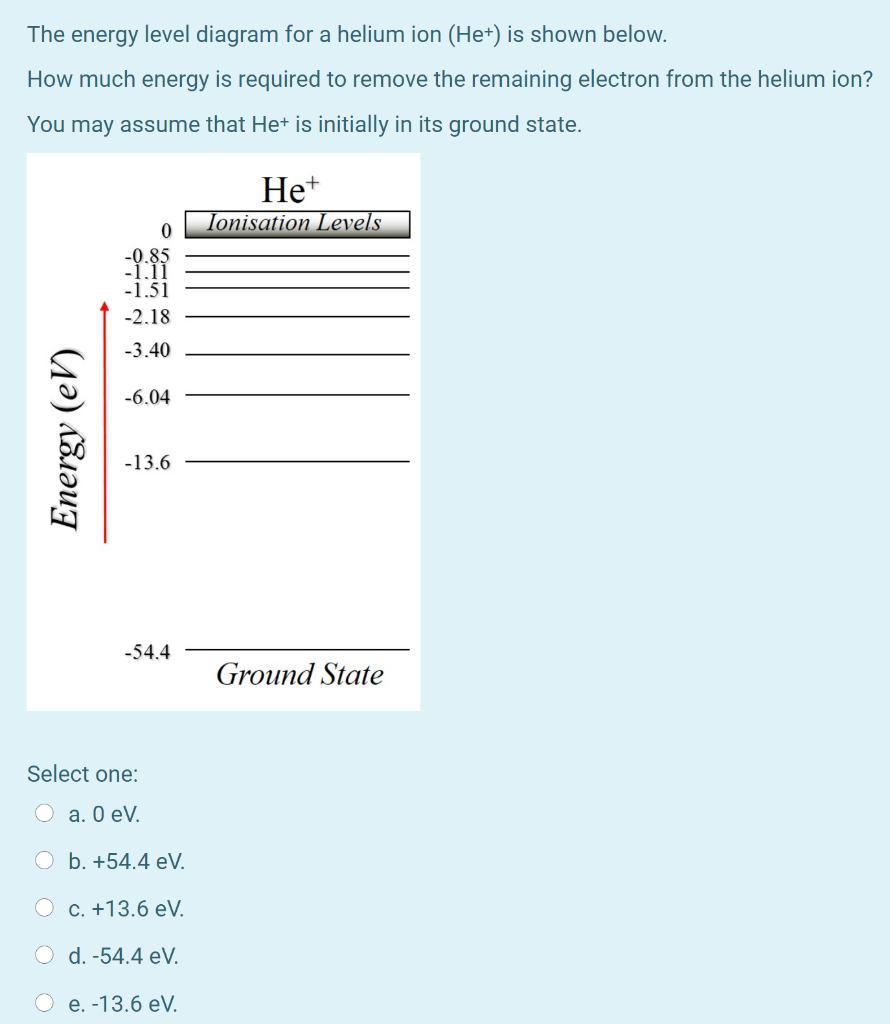
The energy level diagram for a helium ion (He+) is shown below. How much energy is required to remove the remaining electron from the helium ion? You may assume that Het is initially in its ground state. He+ Ionisation Levels 0 -0.85 -1.11 -1.51 -2.18 -3.40 -6.04 Energy (en) -13.6 -54.4 Ground State Select one: O a. O eV O b. +54.4 eV. O c. +13 ...
Energy Levels of Helium Introduction In this experiment you will verify the quantization of energy levels in the helium atom, and measure the energy levels, using a critical potential tube. All electrons within atoms are restricted by quantum theory to discrete energy levels as originally predicted by Bohr’s model of the atom.
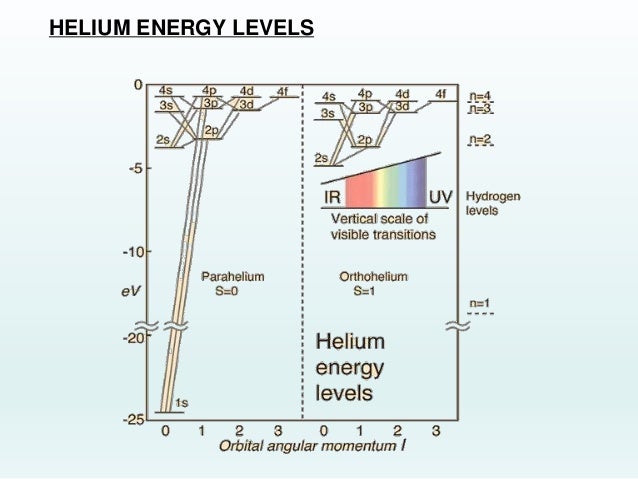
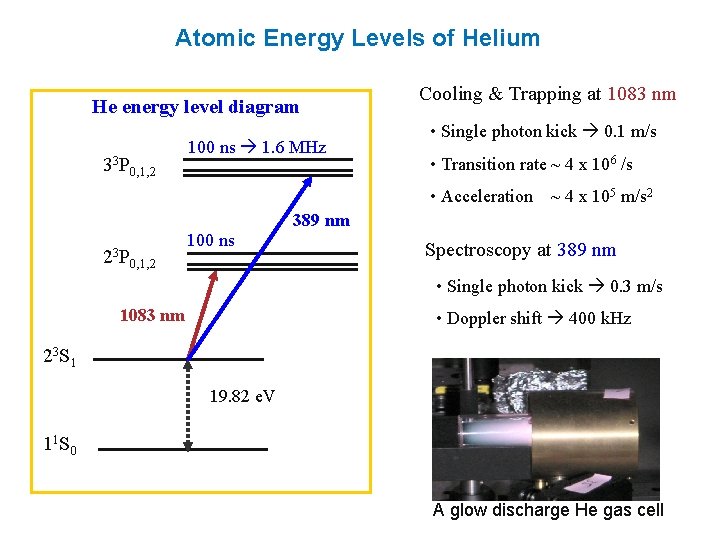
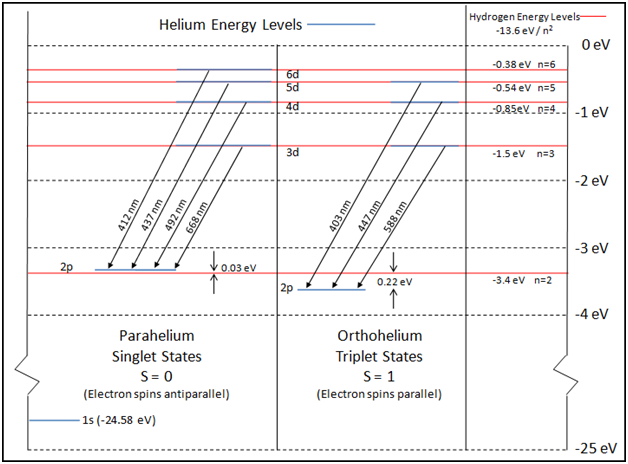
An energy level diagram for helium is shown in Figure 1. The singlet states are on the left and the triplet states are on the right. Note that "singlet" states are those in which the two helium electrons have opposite spin, while "triplet" states are those whose electrons have the same spin. Figure 1.
Helium is a chemical element with atomic number 2 which means there are 2 protons and 2 electrons in the atomic structure. Helium energy level diagram Helium ground state 1s 2 1S0. The lewis dot structure for helium he is he. We Have got 9 picture about Helium Atom Images images photos pictures backgrounds and more.
Helium energy level diagram Helium ground state 1s 2 1S0 Helium excited states 1snl 1LJ or 3LJ. He 1s2p fine structure, compared to 1s2s level position Units: due to variety of energy scales in atomic physics, you may see energies displayed in
While the energy level diagram of hydrogenwith its single electron is straightforward, things become much more complicated with multi-electron atomsbecause of the interactions of the electrons with each other. The electron energy levels for a helium atomdemonstrate a number of features of multi-electron atoms. The labeling of the levels follows the spectroscopic notation.
Jun 11, 2020 · What is the orbital diagram for Helium? Helium only has 2 electrons and therefore it has a configuration of 1s2. Because the 1s orbital is full with 2 electrons and any additional electrons would go in a new energy level. The electron configuration for Helium shows a full outer shell and is Helium is therefore called a Nobel Gas.
Download scientific diagram | Energy level diagram of helium atom associated with the commonly observed spectrum including the metastable excited energy levels. from publication: Direct evidence ...
The ionization energy of an atom is the energy required to remove the electron completely from the atom. (transition from ground state n = 0 to infinity n = ∞ ). For hydrogen, the ionization energy = 13.6eV. When an excited electron returns to a lower level, it loses an exact amount of energy by emitting a photon.
In the helium energy level diagram, one electron is presumed to be in the ground state of a helium atom, the 1s state. An electron in an upper state can have spin antiparallel to the ground state electron (S=0, singlet state, parahelium) or parallel to the ground state electron (S=1, triplet state, orthohelium).
ABOUT THE VIDEO*****He-Ne laser was the first gas laser fabricated by ALI-JAVAN & his co-workers in 1961.Main features of this laser are:1.I...
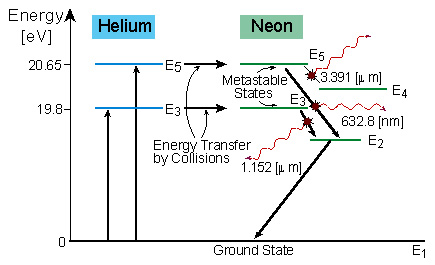
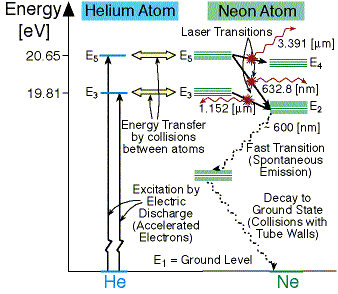
The energy level diagram of helium and neon atom is shown below. he ne laser energy level diagram. energy level diagram of He-Ne laser. When electric discharge occurs in the discharge tube, some of the atoms of the mixture of He-Ne that ionized. The electrons obtained so collide with Helium atom in the ground state or level and hence Helium ...
The figure shows the electron energy level diagram during a collision between a helium and a neon atom, which occurs in helium-neon lasers. A population of electrons is created in the metastable ...
The energy level diagram for He and Ne atoms are shown in Fig2. When an electric discharge passes through the gas, the electron in the discharge tube collide with the He and Ne atoms and excite them to metastable states of energy 20.61 eV and 20.66 eV respectively above the ground state. Some of the excited helium atoms transfer their energy to ...
I discover something: The energy level of Helium is just energy level of Hydrogen plus 10 eV. My hypothesis is that this extra energy comes from the repulsion energy between the 2 electrons of Helium when one in excited state and one in ground state. My approach is to use the Bohr radius. For the ground state one (n=1), it would be a radius ...
Figure 3.4 show the energy level diagram of Helium-Neon laser, with the possible transitions. The mass of the Helium atom is about one-fifth of the mass of the Neon atom. The amount of Helium in the tube is about 6 times the amount of Neon. Thus Helium atoms have more chance to receive energy from the accelerated electrons, and transfer into ...
The molecular orbital energy level diagram of He2 (hypothetical) is given in Fig. Here, Nb = 2 and Na = 2 Bond order = Nb - Na / 2 = 2 - 2 / 2 = 0. As the bond order for He2 comes out to be zero, this molecule does not exist. 3. Nitrogen molecule (N2). The electronic configuration of nitrogen (Z=7) in the ground state is 1s2 2s2 2p1x 2p1y 2p1z .
The energy diagram for helium is shown as here. Notice that there has been a change in the relative energies of the 2s and 2p orbitals. This is an important point that must be addressed at this point. In the hydrogen atom the sublevels in each principle level are degenerate.
Working of He-Ne. It is a four energy level laser system. The electrons produced from electric discharge collide with He and Ne atom and excite them to the higher energy levels He2 and Ne4 at 20.61 eV and 20.66 eV respectively. These two states are metastable so the atoms may stay there for a longer time. They are very close to each other, thus ...
In this quantitative investigation, the total singlet-triplet splitting of the 2p valence electrons of helium was seen to be 0.22 eV + 0.03 eV = 0.25 eV, as is shown in the energy level diagram displayed earlier. Consultation of the NIST Atomic Spectra Database reveals agreement of our value with published values.
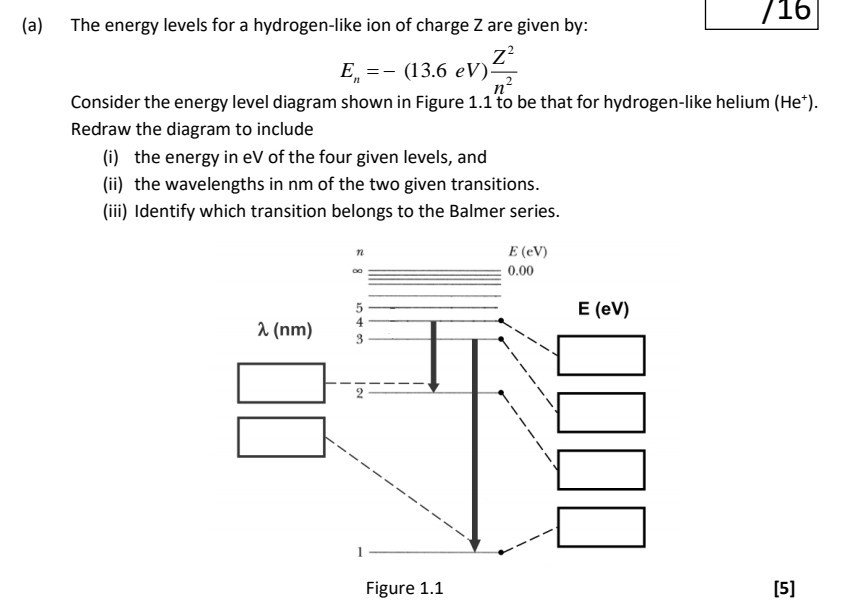
Construct the energy-level diagram (like Fig. 37-26) for (a) He+ ion and (b) doubly ionized lithium Li2+. Solution 9.3 (a) Singly ionized helium is like hydrogen, except that there are two positive charges (Z = 2) in the nucleus. The square of the product of the positive and negative charges appears in the energy term for the energy levels. ...























0 Response to "40 energy level diagram for helium"
Post a Comment