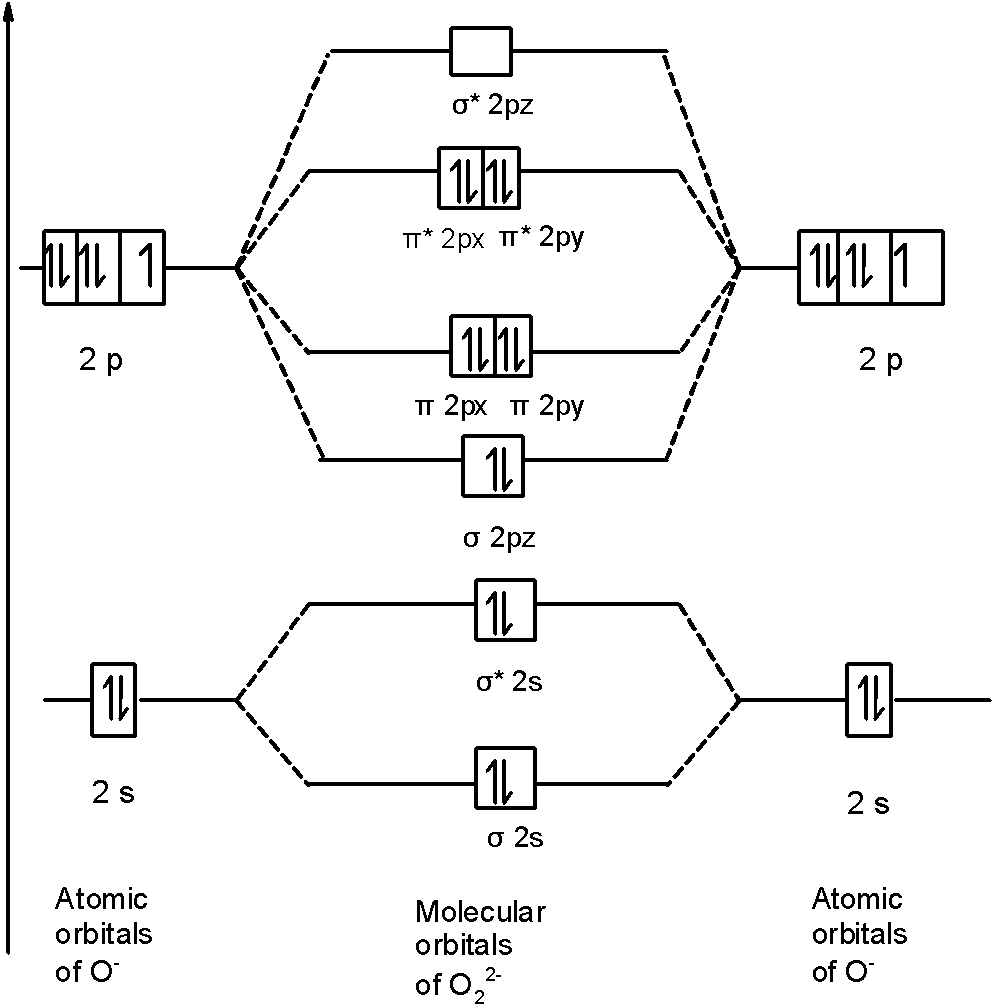
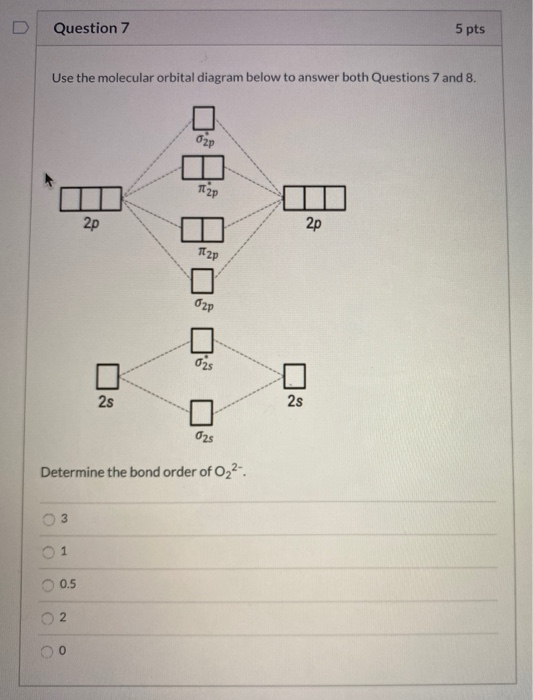
38 o2 2- molecular orbital diagram
Molecular orbital diagrams are diagrams of MO energy levels, shown as short horizontal lines in the center, flanked on the sides by constituent AO energy levels for comparison, with the energy levels ranging from low energy at the bottom to high energy at the top. • Bonding - Review VSEPR and Hybridisation - Linear combination of molecular orbitals (LCAO), bonding / antibonding - Labelling of molecular orbitals (MOs) (σ, π and g, u) - Homonuclear diatomic MO diagrams - mixing of different AO's - More complex molecules (CO, H2O ….)
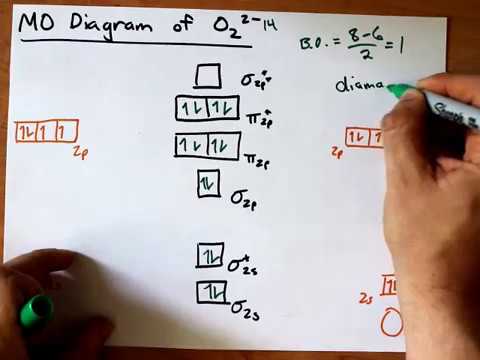
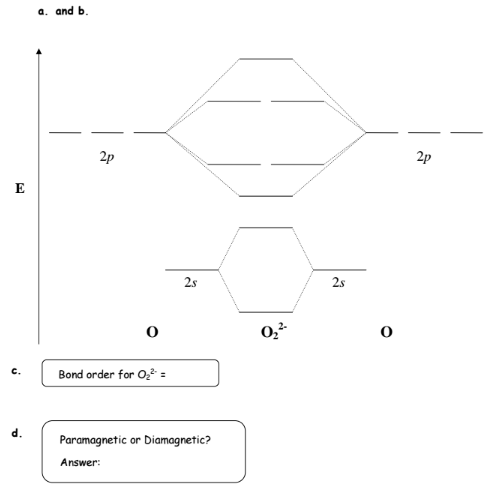
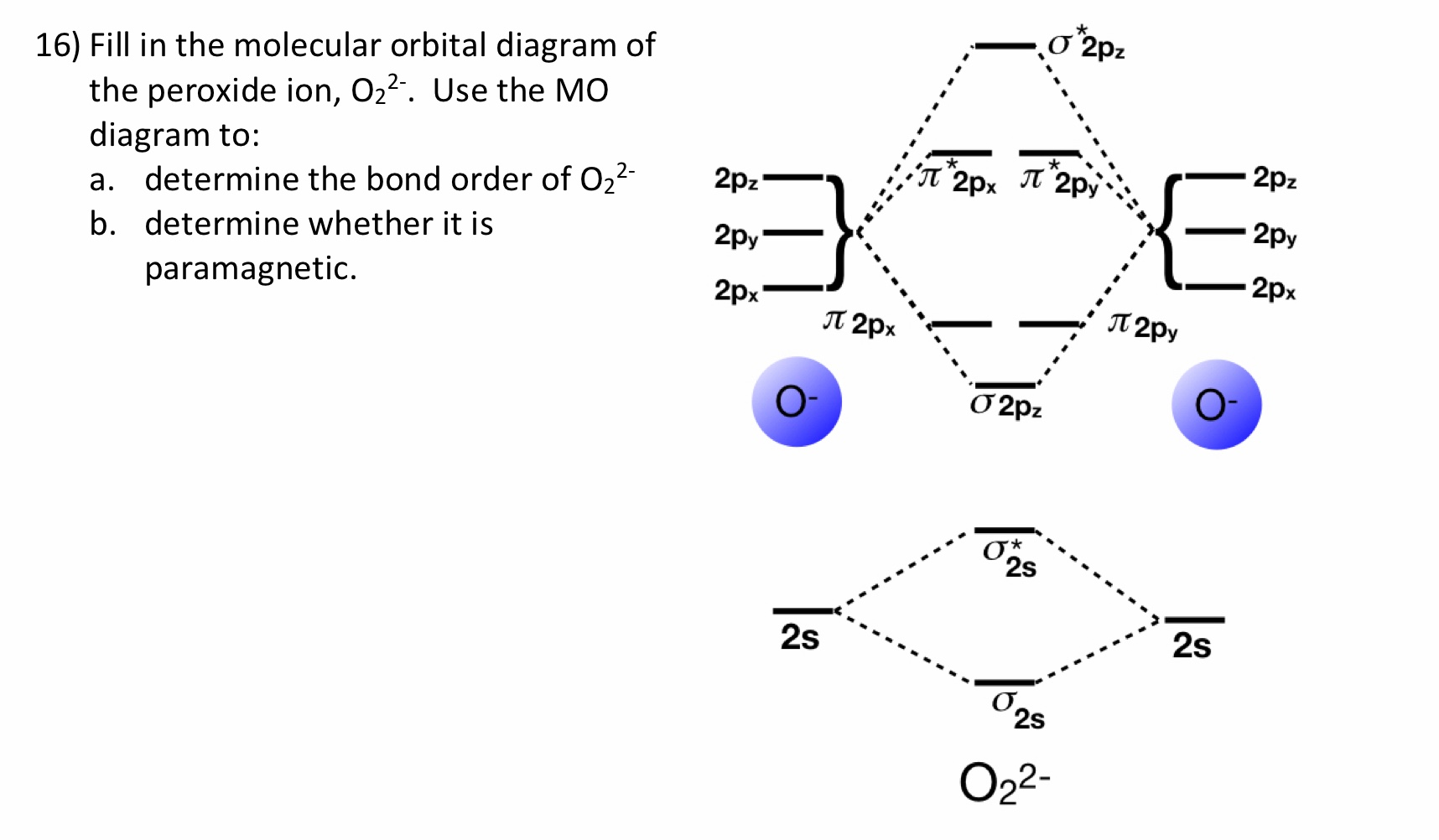
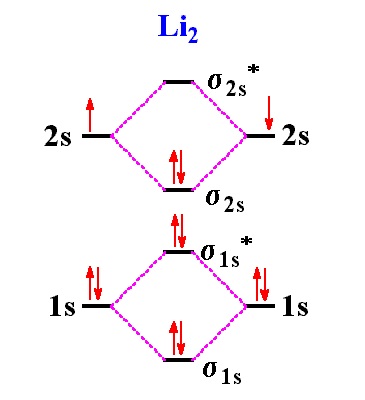
Bonding order is 2 and it is paramagnetic. The two hydrogen 1s orbitals are premixed to form a 1 σ and b 2 σ mo.
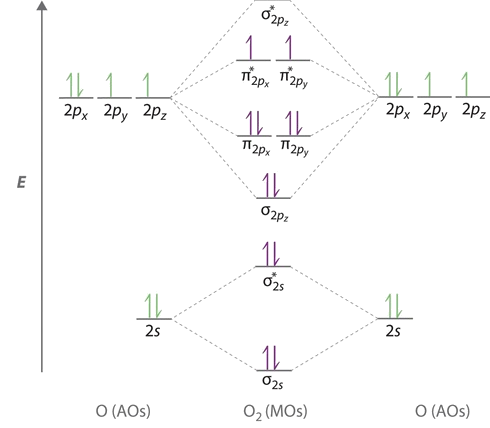
O2 2- molecular orbital diagram
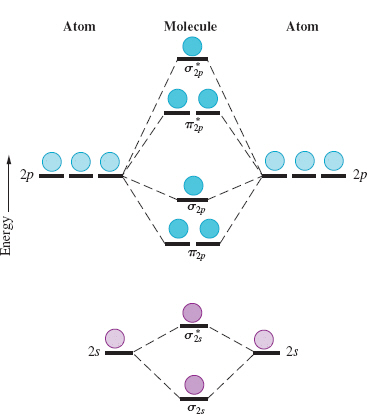
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. • Because the energy of the two electrons is lower than the energy of the individual atoms, the molecule is stable. Figure 9.26: (a) The molecular orbital energy-level diagram for the H2 molecule. (b) The shapes of the molecular orbitals are obtained by squaring the wave functions for MO1 and... The bonding molecular orbital concentrates electrons in the region directly between the two nuclei. The molecular orbital diagram for an O2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions between the 2s and 2p valence orbitals.
O2 2- molecular orbital diagram. Figure 9-2 Molecular orbital (MO) diagram for the combination of the 1s atomic orbitals on two identical atoms (at the left) to form two MOs. One is a bonding orbital, 1s (blue), resulting from addition of the wave functions of the 1s orbitals. Remember: When two oxygen atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals. They are flipped... no two molecular orbitals for any molecule ever have the same energy. which of the following statements concerning the molecular orbital energy level diagrams for first and second period homonuclear diatomic molecules is FALSE. the diagram for O2, F2, and Ne2 molecules has the... The diagram shows how the molecular orbitals in lithium hydride can be related to the atomic orbitals of the parent atoms. Notice that the relative energies of the 2p-derived σ and π bonding molecular orbitals are reversed in O2 and F2. This is attributed to interactions between the 2s orbital each atom...
Wiring Diagram: 9 Molecular Orbital Diagram For O2 (Ivan Holloway). This different from Nitrogen, where it's the. Learn vocabulary, terms and more with flashcards, games and other study tools. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. For a diatomic molecule... Chapter 2 - Molecular Orbital Theory Big-picture: Now that we understand aspects of molecular structure, we Learning goals: • Be able to construct molecular orbital diagrams for homonuclear diatomic, heteronuclear diatomic, homonuclear triatomic, and heteronuclear triatomic molecules. • Molecular orbital diagrams are diagrams of MO energy levels, shown as short horizontal lines in the center. Atomic orbitals (AO) energy levels are shown for comparison. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels. Molecular orbital : A molecule in which all the electrons are paired, is called diamagnetic. | Online Chemistry tutorial IIT, CBSE Chemistry, ICSE Chemistry, engineering and medical chemistry entrance exams Molecular orbital diagram of C2 molecule : Number of electrons in C2 molecule = 12.
Bonding order is 2 and it is paramagnetic. Molecular orbital theory describes the distribution of electrons in molecules in much the same w... Draw the Molecular Orbital Diagram of H2+, O2- and N2 molecules and calculate their bond orders as well as tell about their properties, whether they are paramagnetic or reference to H2O, CO2 and C2H2. 13. Write a note on chemical shift. 14. What is stretching and bending vibrations of molecule? This video shows the construction of a molecular orbital (MO) diagram for the diatomic molecule, O2, using the valence electrons of each oxygen. Oxygen's paramagnetism (unpaired electrons) is seen the MO diagram. FYI: I use the x axis as the horizontal axis (because I've always done that - it's what... Information from the mo diagram justify o2s stability and show that its bonding order is 2. Printable o2 molecular orbital diagrams are ava...
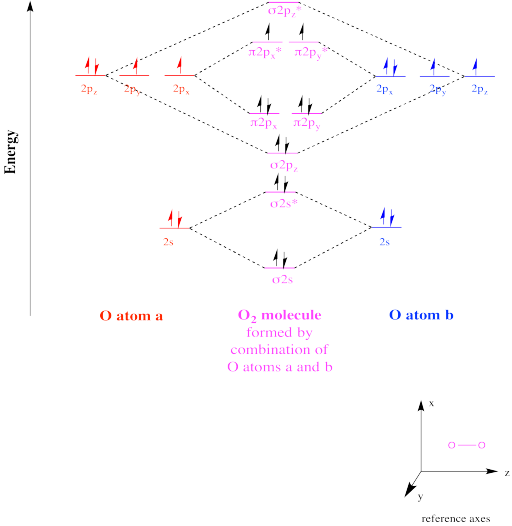
As it can be seen from the MOT of O2 , The electrons in the highest occupied molecular orbital are unpaired therefore it is paramagnetic in nature.
From that diagram, you can then easily fill out what the O2- and O2+ MO diagrams should be—and that is in the second photo I included. The first photo is straight from a 2006 edition Pearson general chemistry textbook, and it shows you what the molecular orbital (MO) diagram for O2 is.
Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting electrons from the diagram for the neutral molecule. Figure 8.40 The molecular orbital energy diagram for O2 predicts two unpaired electrons. We calculate the bond order as.
Berkas Valence Orbitals Of Oxygen Atom And Dioxygen Molecule Diagram Svg Wikipedia Bahasa Indonesia Ensiklopedia Bebas
Valence bond (VB) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons.
You should know that molecular orbital diagrams are used to deduce magnetic properties of a molecule; they also help us to find out the bond order of the molecule. Complete step by step answer: First let us understand the concept of molecular orbital theory. On a very general basis, electrons...
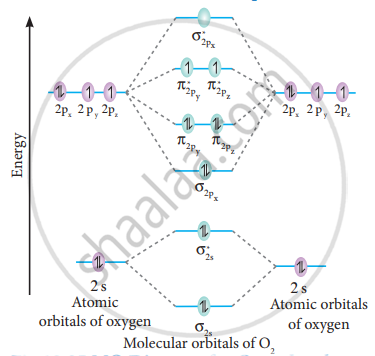
Draw The M O Diagram For Oxygen Molecule Calculate Its Bond Order And Show That O2 Is Paramagnetic Chemistry Shaalaa Com
When determining the molecular orbital (MO) configuration of a homonuclear diatomic molecule like "O"_2 or "F"_2, first, we should determine the So, write the MO configuration based on this diagram. Start with the lowest-energy orbital, and indicate the electrons in each kind of orbital, just like atomic...
Write The Electronic Configuration Energy Level Diagram For The Molecular Orbitals Of Oxygen Molecule O2 Sarthaks Econnect Largest Online Education Community
Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular Orbital Theory. Valence Bond Theory proposes that electrons are localized between two atoms.
A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row

How To Draw Molecular Orbital Diagram Of O2 Simplest Trick Chemistry Best Online Free Chemistry Class 9 12
Molecular Orbital Theory. I'm having a lot of trouble with this stuff. I don't really know how to start these questions (such as how to draw a correlation diagram) Feel free to ask clarification questions. I'll use your example of O2-. An orbital correlation attempts to show how the atomic orbitals belonging to...
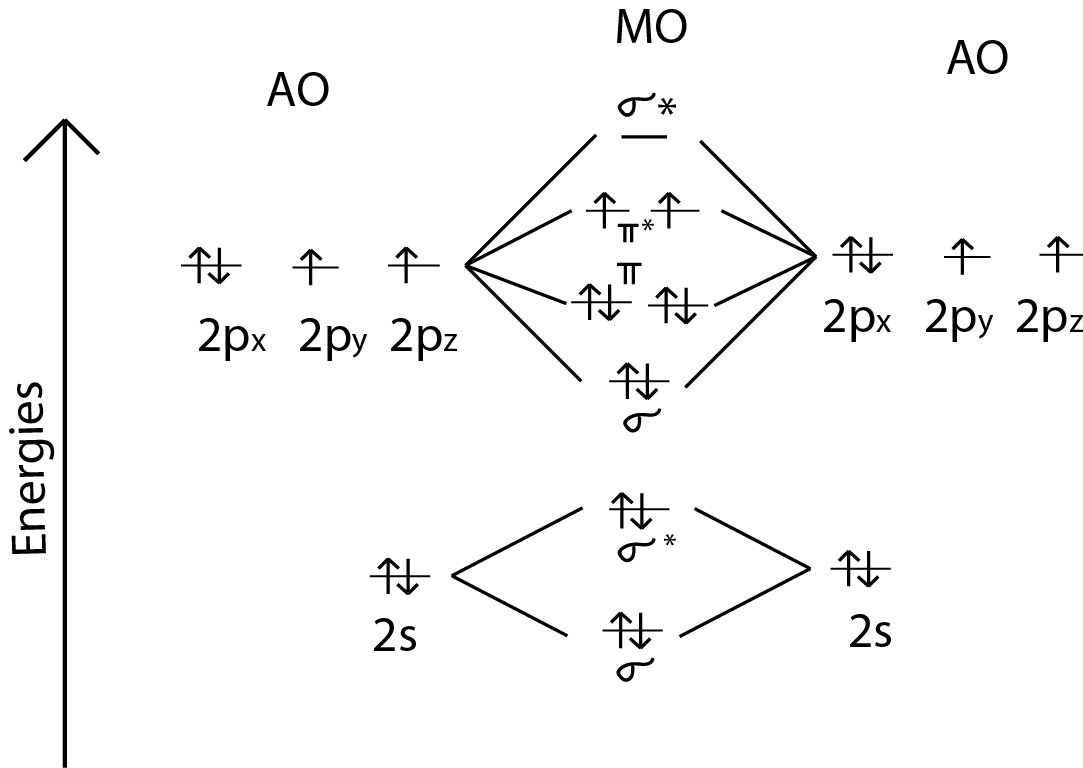
The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. The MOs for the valence orbitals of the second period are shown in Figure 11. Looking at Ne2 molecular orbitals, we see that the order is consistent with the generic diagram...

Molecular Orbital Theory Homodiatomics Use The Molecular Orbital Model To Fully Describe The Bonding In O2 O2 O2 And O22 Determine Which Of The Following Statements Are True And Which Are
The molecular orbital diagram representing this order of energy levels is shown in fig. This kind of mixing of orbitals or symmetry interaction is not applicable for O2 and F2 molecule formation because of larger energy gap between 2s and 2p orbitals for these atoms.
The bonding molecular orbital concentrates electrons in the region directly between the two nuclei. The molecular orbital diagram for an O2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions between the 2s and 2p valence orbitals.
• Because the energy of the two electrons is lower than the energy of the individual atoms, the molecule is stable. Figure 9.26: (a) The molecular orbital energy-level diagram for the H2 molecule. (b) The shapes of the molecular orbitals are obtained by squaring the wave functions for MO1 and...
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.

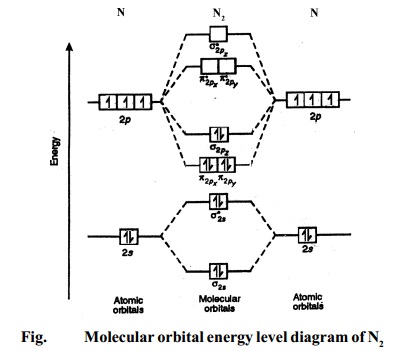
Draw The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Paramagnetic N22 B22 Homeworklib

With The Help Of Molecular Orbital Theory Draw The Molecular Orbital Energy Level Diagram For N 2 Molecule Also Calculate The Bond Order And Predict The Magnetic Behaviour
Write The Electronic Configuration Energy Level Diagram For The Molecular Orbitals Of Oxygen Molecule O2 Sarthaks Econnect Largest Online Education Community

Pleaseshow Me The Energy Level Diagrams Of O2 O2 O2 2 Chemistry Chemical Bonding And Molecular Structure 7027301 Meritnation Com

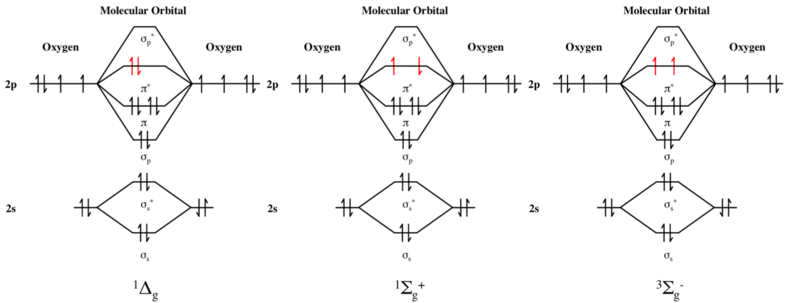








0 Response to "38 o2 2- molecular orbital diagram"
Post a Comment