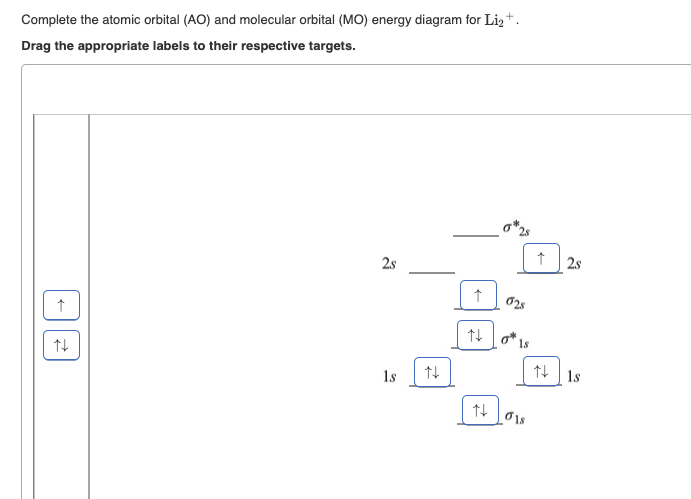
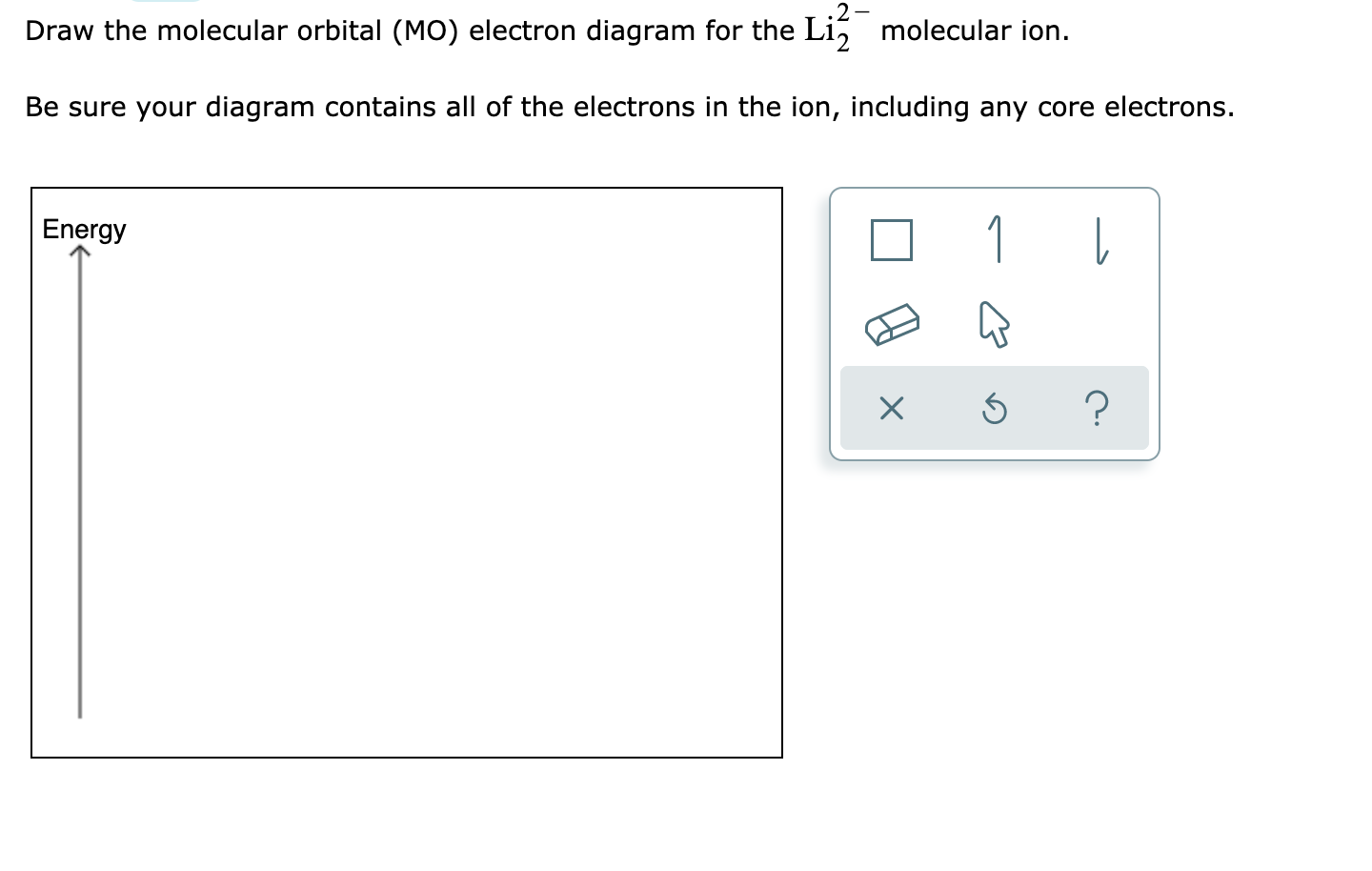
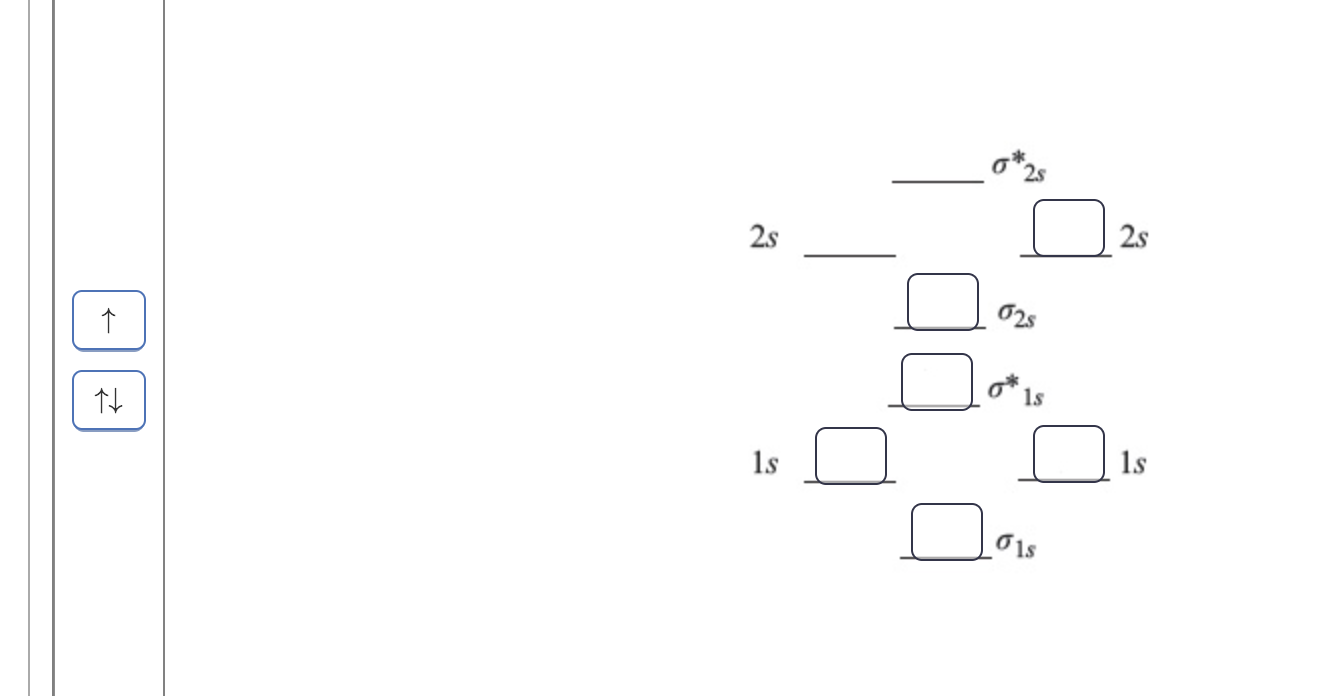
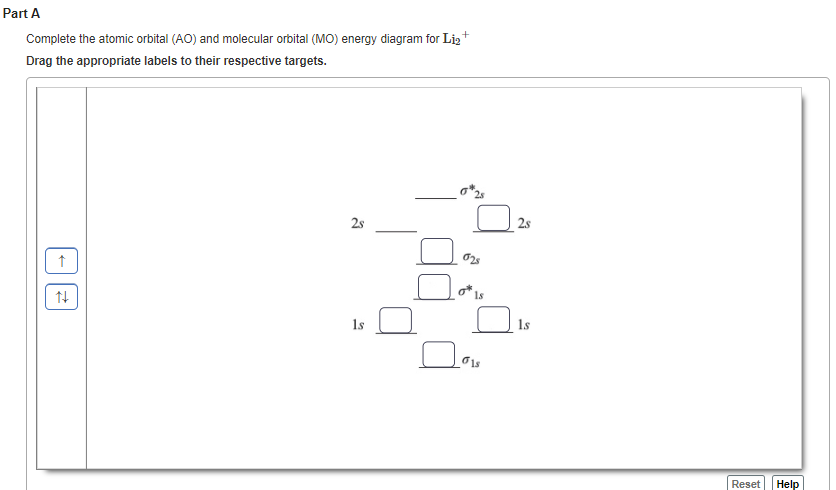
38 complete the atomic orbital (ao) and molecular orbital (mo) energy diagram for li2+.
FREE Answer to 1)Complete the atomic orbital (AO) and molecular orbital (MO) energy diagram for Li2+ and Li2-1 answer · Top answer: Molecular orbital theory is also used to explain bonding in molecules using linear combination of atomic orbitals. According to molecular orbital theory, ... 17:44This lecture is for the JEE/ NEET Aspirants and for all those who are interested.Do follow me on Unacademy to ...20 Sep 2018 · Uploaded by Chemistry Enthusiasts by Megha Khandelwal
11 Dec 2019 — Get the detailed answer: complete the atomic orbital (ao) and molecular orbital (mo) energy diagram for li2+.1 answer · Top answer: The Molecular Orbital Theory states that each atom tends to combine together to form molecular orbitals. In MOT, electrons in a molecule are not assigned ...
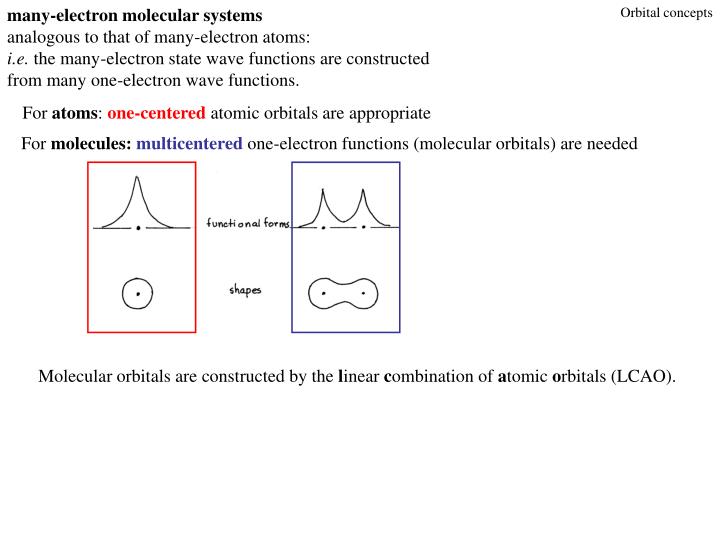
Complete the atomic orbital (ao) and molecular orbital (mo) energy diagram for li2+.
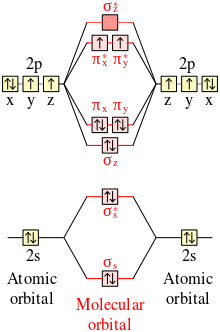
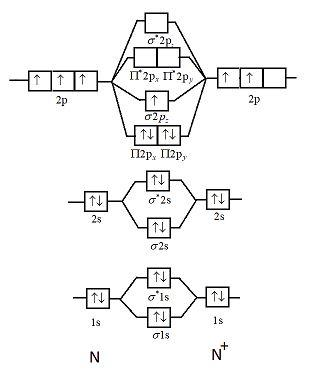
Complete the atomic orbital ao and molecular orbital mo energy diagram for li2. Li0 2s1 overlap of the two 2s aos results in a σ bonding mo that is lower in energy than the constituent 2s aos and an antibonding σ mo that is at a higher energy than the 2s aos. Complete the atomic orbital ao and molecular orbital mo energy diagram for li2. It describes the formation of bonding and antibonding molecular orbitals from the combination of atomic. Use simple lcao linear combination of atomic orbitals mo theory. Each mo can hold two es and hence for. 1 answerProblem: Draw the molecular orbital (MO) energy diagram for Li2+. FREE Expert Solution. The total number of valence electrons present in Li2+ is:.
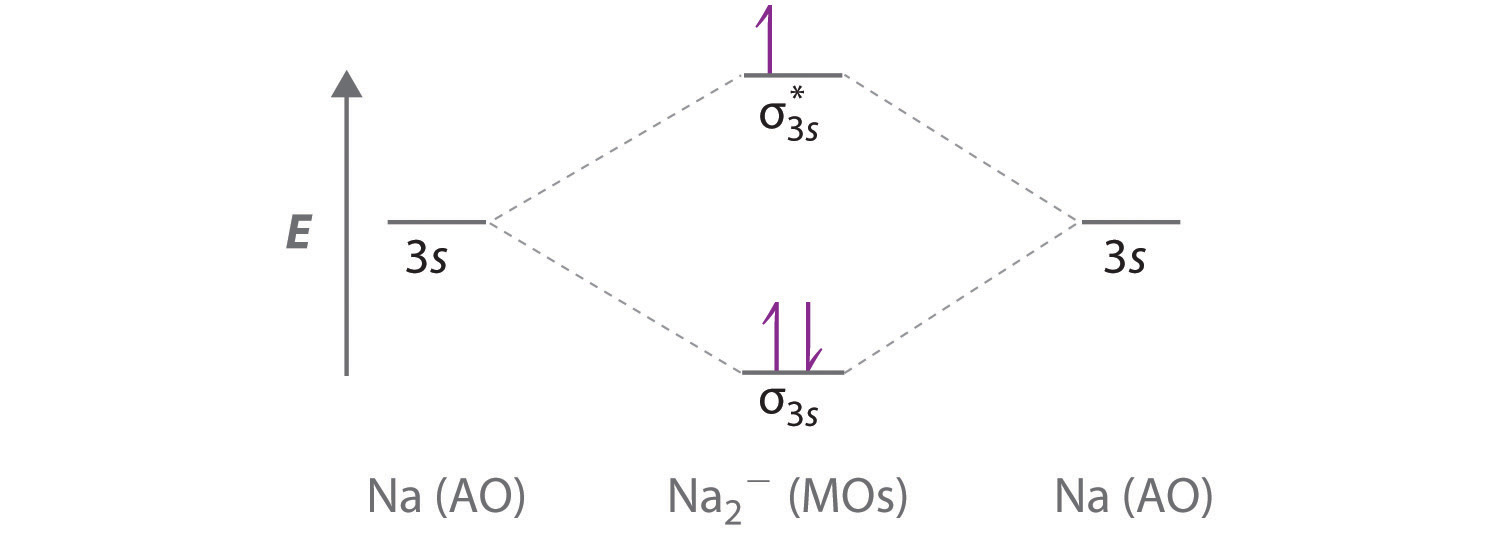
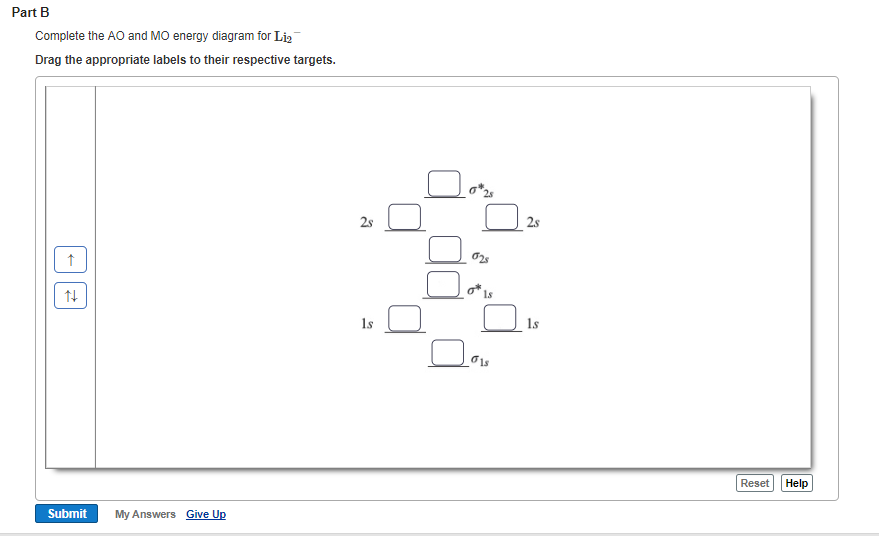
Complete the atomic orbital (ao) and molecular orbital (mo) energy diagram for li2+.. Complete the atomic orbital ao and molecular orbital mo energy diagram for li2 . Removal of one e from li2 to give li2 results in an mo scheme of σσ0. The same method can be applied to other diatomic molecules but involving more than the 1 s atomic orbitals. Answer to 1complete the atomic orbital ao and molecular orbital mo energy diagram for ... 1)Complete the atomic orbital (AO) and molecular orbital (MO) energy diagram for Li2+ and Li2-. Best Answer. This is the best answer based on feedback and ratings. 89% (18 ratings) 3 Aug 2018 — Here we consider the molecular orbital diagram (MO) of Li2 : ... for each electron in a bonding MO, it adds 0.5 to the bond order, ...1 answer · No... it's the other way around. Li2 is more stable than Li+2, because the bond is (hypothetically) stronger (probably gas-phase). Here we consider ... Complete the AO and MO energy diagram for Li2−. Assume the left AO comes from Li− and the right AO comes from Li. Question: Complete the AO and MO energy diagram for Li2−. Assume the left AO comes from Li− and the right AO comes from Li.
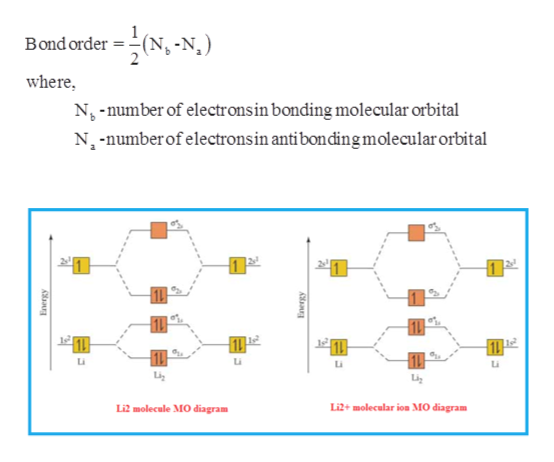
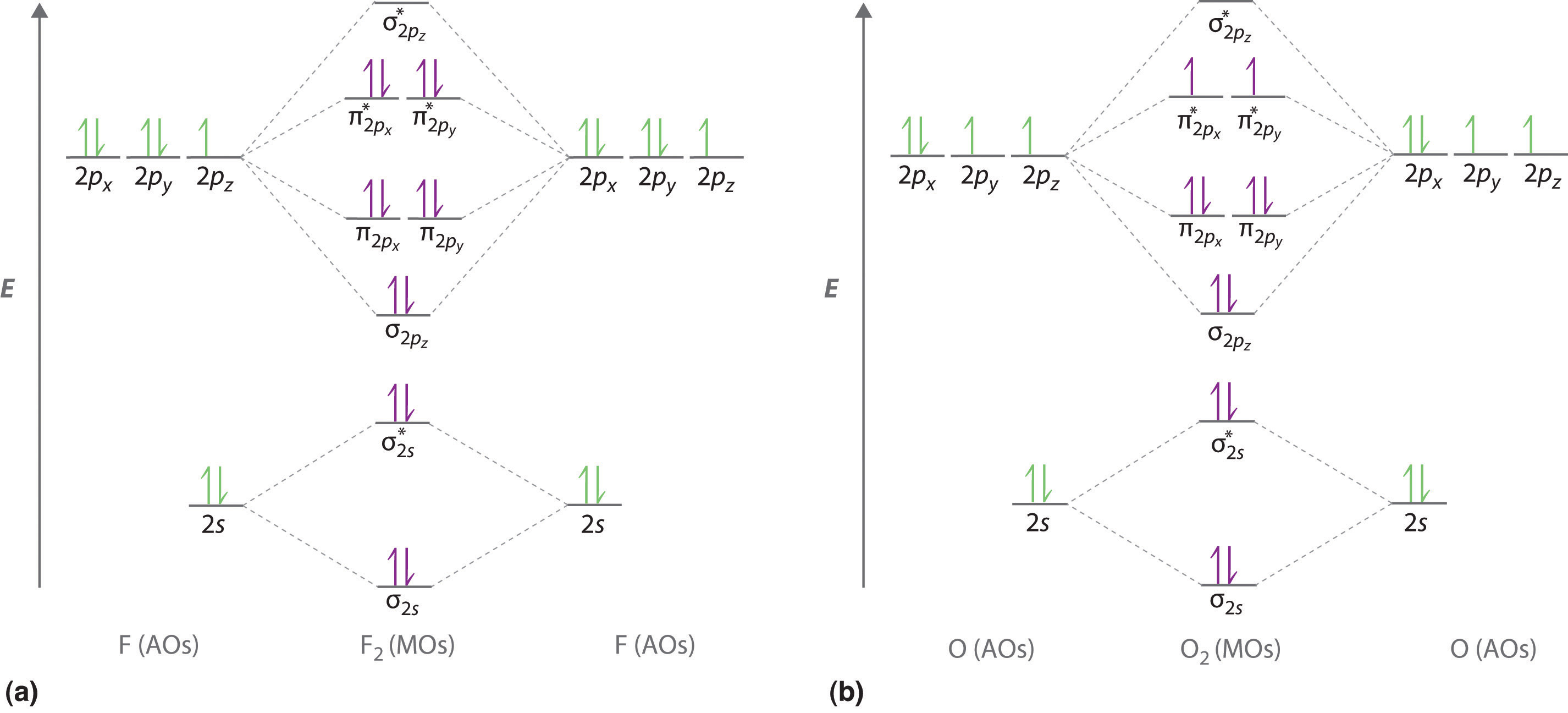
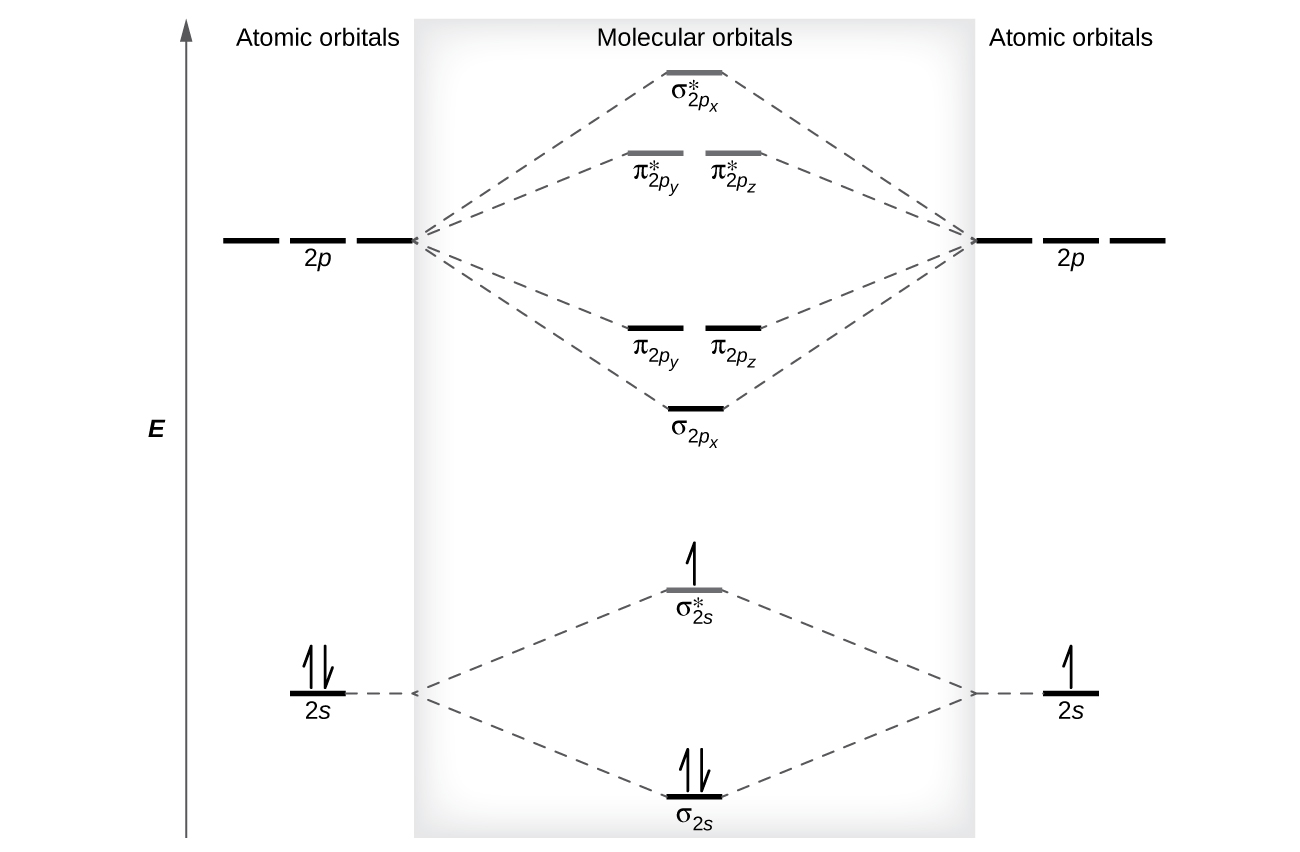
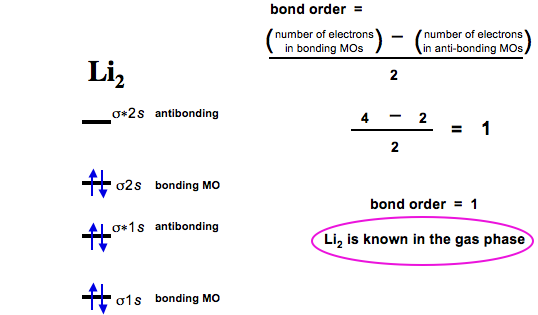
Academia.edu is a platform for academics to share research papers. 3:29Full first shell:sigma-1s and sigma1s* are both full.sigma-2s is full; sigma-2s* is empty.Bond order 1 = single bond.4 May 2020 · Uploaded by chemistNATE 17:57This chemistry video tutorial provides a basic introduction into molecular orbital theory. It describes the ...13 Jun 2020 · Uploaded by Digital Kemistry Part b complete the ao and mo energy diagram for li2 drag the appropriate labels to their respective targets. The bond order for li2 is 1. A molecular orbital mo energy level diagram appropriate for homonuclear diatomic molecules from li2 to n2 is shown below. The mo bond order is the main factor controlling the internucelar distance.
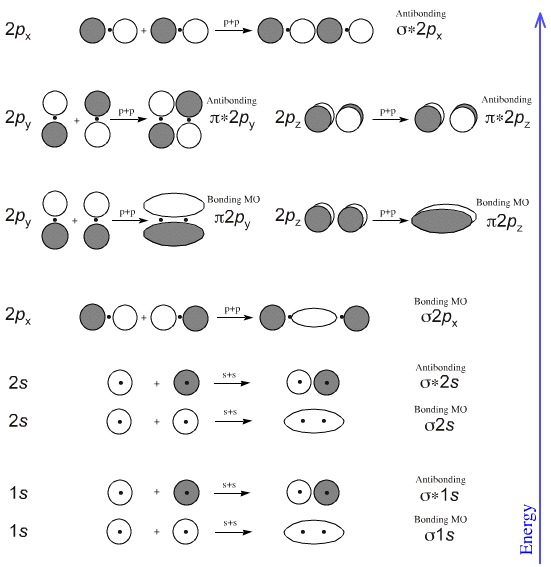
For the ion li2. Molecular orbital diagrams of diatomic molecules. 1complete the atomic orbital ao and molecular orbital mo energy diagram for li2 and li2 1complete the atomic orbital ao and molecular orbital mo energy diagram for li2 and li2 best answer. If the phase changes the bond becomes a pi bond π bond. Bond order is 3 and it is ... Molecular orbital and. Complete the atomic orbital ao and molecular orbital mo energy diagram for li2. This mo is called the bonding orbital and its energy is lower than that of the original atomic orbitals. Then we rank them in order of increasing energy. B calculate the bond order. Explain the terms. Molecular orbital and. Complete the atomic orbital ao and molecular orbital mo energy diagram for li 2. If the phase changes the bond becomes a pi bond π bond. B calculate the bond order. Before we can draw a correlation diagram for b2 we must first find the in phase and out of phase overlap combinations for borons atomic orbitals. Complete the atomic orbital ao and molecular orbital mo energy diagram for li2. Please use a different device to submit your solution for grading. Chem 344 Homework 9 Due Friday Apr 11 2014 2 Pm The same method can be applied to other diatomic molecules but involving more than the 1s atomic orbitals.
1 answerProblem: Draw the molecular orbital (MO) energy diagram for Li2+. FREE Expert Solution. The total number of valence electrons present in Li2+ is:.
Complete the atomic orbital ao and molecular orbital mo energy diagram for li2. It describes the formation of bonding and antibonding molecular orbitals from the combination of atomic. Use simple lcao linear combination of atomic orbitals mo theory. Each mo can hold two es and hence for.
Complete the atomic orbital ao and molecular orbital mo energy diagram for li2. Li0 2s1 overlap of the two 2s aos results in a σ bonding mo that is lower in energy than the constituent 2s aos and an antibonding σ mo that is at a higher energy than the 2s aos.

Complete The Atomic Orbital Diagram For The Ground State Electronic Configuration Of Chlorine Answer Bank Energy Homeworklib

1 Complete The Atomic Orbital Ao And Molecular Orbital Mo Energy Diagram For Li2 And Li2 Homeworklib















0 Response to "38 complete the atomic orbital (ao) and molecular orbital (mo) energy diagram for li2+."
Post a Comment