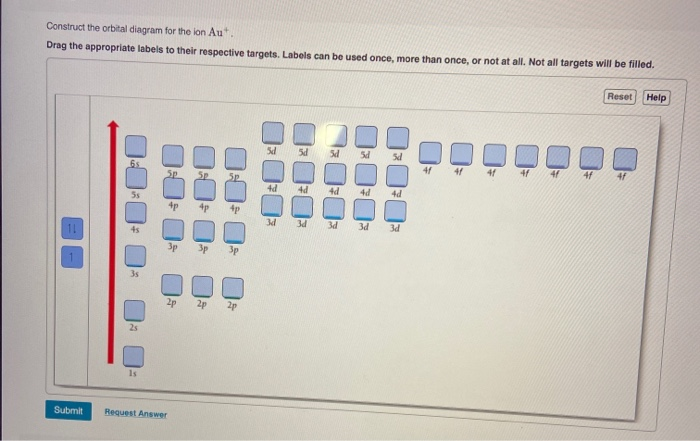
41 enter the orbital diagram for the ion au+.
What do some recovery support services provide Answer. Option (2) is the correct answer. By creating job opportunities and helping them with employment is a best support services. By creating employme refferal and employment will them to reach their aims. Construct the orbital diagram of the f ion - Soetrust Construct the molecular orbital diagram for he2; Molecular orbital diagram of h2; Use the molecular orbital diagram shown to determine which… Write orbital diagram for Au+? Construct a graph corresponding to the stone's vertical… Given ab, explain how to construct a square with sides of…
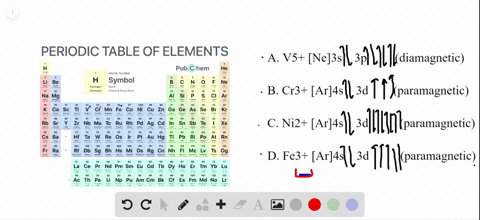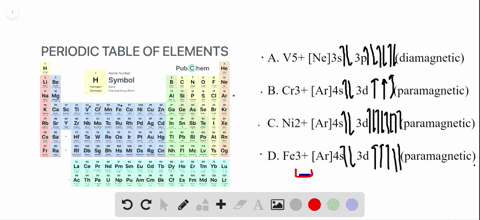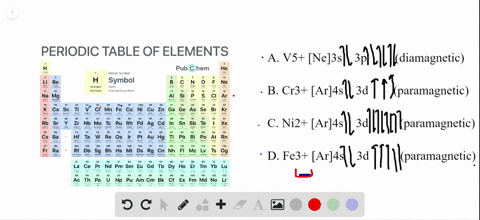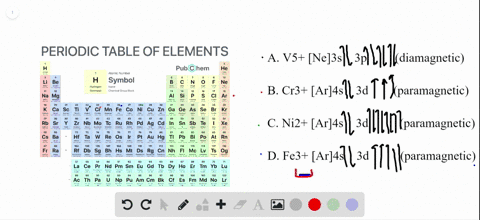
Write orbital diagram for Au+? Since Au+ has lost one electron and the numbers (or arrows in the diagram) represent the electrons, its configuration is: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10. So there will not be the 6s orbital.

Enter the orbital diagram for the ion au+.
Inorganic Chemistry Flashcards - Quizlet Simplest whole number ratio of the ions that yields electrical neutrality. Naming Rules 1. Cation first, then anion. 2. For monatomic cations, give the elements name. 3. For monatomic anions, affix an -ide ending to the stem of the element's name. 4. What is the electron configuration of Au+? | Socratic [Xe] 4f^14 5d^10 The atomic number of Au is 79. Therefore, its configuration is: 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^6 4d^10 5s^2 5p^6 4f^14 5d^10 6s^1 or, [Xe] 4f^14 5d^10 6s^1 For Au^+, one electron is removed from the outermost 6s orbital, making the configuration, [Xe] 4f^14 5d^10 SOLVED:Write orbital diagrams for each ion and determine ... Zr2+ Answer Write orbital diagrams for each ion and determine if the ion is diamagnetic or paramagnetic. a. Cd2+ b. Au+ c. Mo3+ d. Zr2+ Discussion You must be signed in to discuss. Video Transcript Chapter three problems, seventy eight says to right orbital diagrams for several ions and determined at those ions. Our dia magnetic appear magnetic.
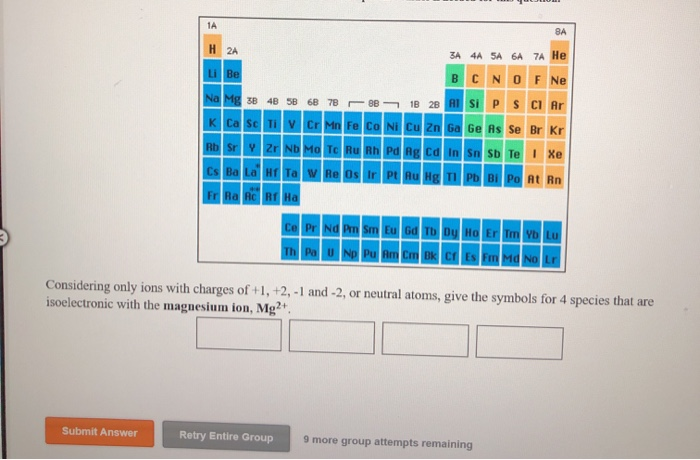
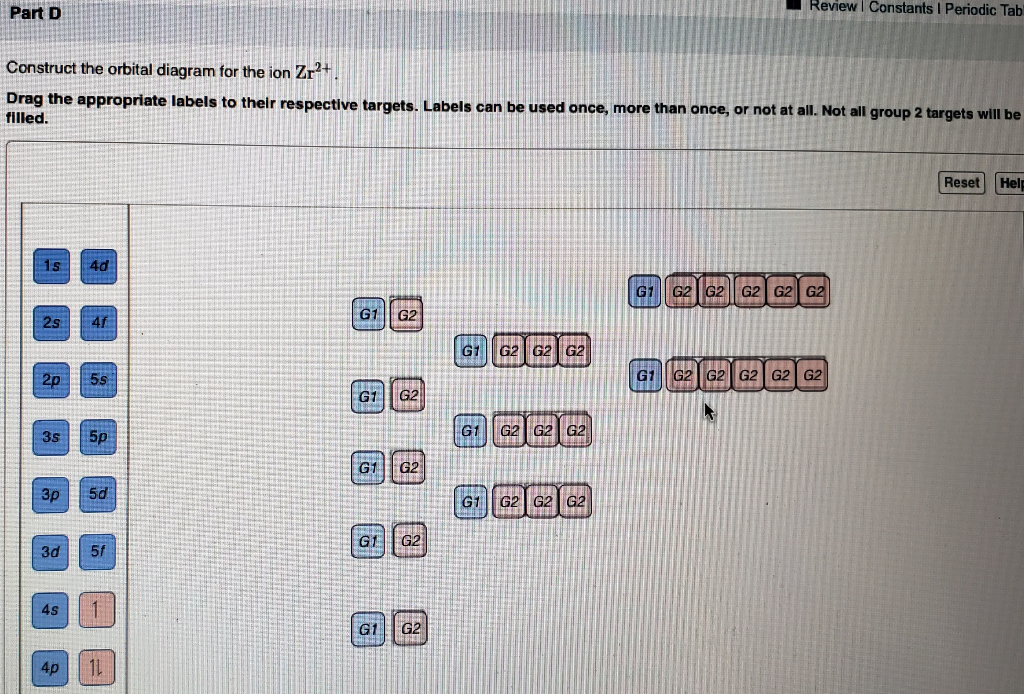
Enter the orbital diagram for the ion au+.. 39 construct the orbital diagram of the f ion - Diagram ... Construct the orbital diagram of the f ion. How to Do Orbital Diagrams - Sciencing The first number is the principal quantum number (n) and the letter represents the value of l (angular momentum quantum number; 1 = s, 2 = p, 3 = d and 4 = f) for the orbital, and the superscript number tells you how many electrons are in that orbital. Solved Enter the orbital diagram for the ion Cd2+Cd2 ... This problem has been solved! See the answer Enter the orbital diagram for the ion Cd2+Cd2+. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all group 2 targets will be filled. Reset Help 1 s Group 1 1 s 4 d Group 1 4 d 2 s Group 1 2 s 4 f Group 1 4 f 2 p Group 1 2 p 5 s ( Jesus Said If You Lean On Me Lyrics, Helen Miller I Won ... G-Eazy londonchinatown.org - I typical ItIf i say that shit climate I typical it She phone call me, I display screen it, I"m only fuckin" if it"s convenient You lie on pussy, that"s weak shit, we pass pussy "round, that"s G shit. Edward Hawkins - oh Happy day londonchinatown.orgWhen jesus washed (when jesus washed) ... (Get Answer) - 1. Enter the orbital diagram for the ion ... 1. Enter the orbital diagram for the ion Cd2+. Use the buttons at the top of the tool to add orbitals. Add them in order of increasing orbital energy. Click within the orbital to add electrons. 2. Enter the orbital diagram for the ion Au+. Use the buttons at the top of the tool to add orbitals. Add...
40 enter the orbital diagram for the ion cd2+. - Diagram ... Enter the orbital diagram for the ion Cd2+ Drag ... 1. Enter the orbital diagram for the ion Cd2+. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all group 2 targets will be filled. 2. Enter the orbital diagram for the ion Au+. 3.Construct the orbital diagram for the ion Mo3+. Orbital Diagram For Au+ - Wiring Diagrams Answer to Write orbital diagram for Au+. Draw an Molecular Orbital energy diagram and predict the bond order of L 2. Using a partial orbital diagram, show . Electron Configuration Electron Configuration Watch later Watch on Electron Configuration, [Xe] 4f14 5d10 6s1. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1. What is the orbital diagram for Au? - Answers The orbital diagram for gold starts with the base [Xe], which is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6. The outer shells are 6s2 5d9. Enter The Orbital Diagram For The Ion Au+., Write Orbital ... ENTER THE ORBITAL DIAGRAM FOR THE ION AU+. Au Electron Configuration (Gold) Electron Configuration The purpose of introducing quantum numbers has been to show that similarities in the electron arrangement or electron configuration lead to the similarities and differences in the properties of elements.
Orbital Diagram For Au+ - schematron.org Figure Write orbital diagram for Au+. Use the buttons at the top of the tool to add orbitals. Add them in order of increasing orbital energy. Click within the orbital to add electrons. SOLVED:Enter the orbital diagram for the ion Cd2+Cd2 ... To do so, we will need to recall (1) the shapes of bonding and antibonding σ and π molecular orbitals, ( 2 ) that each orbital can contain a maximum of two electrons, (3) that molecular oxygen has 16 electrons in total, and ( 4 ) that the two unpaired electrons in oxygen occupy separate degenerate (equal-chergy) orbitals. Chapter 8 Chemistry Homework Flashcards - Quizlet Enter the orbital diagram for the ion Au+ ‣ When an element is a cation (+) you REMOVE electrons. ‣ Electrons are generally removed from the "s" sub-level 1.) Remove one electron from 5s1 ANSWER: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 Enter the orbital diagram for the ion Mo3+ ‣ When an element is a cation (+) you REMOVE electrons. What is the electron configuration of Cr 3+? - Socratic.org Note that it is 4s13d5 and not 4s23d4 because a half filled d orbital is more stable than a partially filled d orbital. However, the chromium ion Cr3+ possesses 24e− −3e− = 21e− due to the loss of 3 of its electrons. Thus, the electron configuration of Cr3+ is: Cr3+:1s22s22p63s23p64s03d3. Answer link.
7. Mount Saint Helens is an example of a volcano. A ... Click here 👆 to get an answer to your question ️ 7. Mount Saint Helens is an example of a volcano. A.composite B.shield C.cinder cone D.Hawaiian
I'M GIVING 80 POINTS!!!!! IT HAS TO BE ... - Brainly.com I'M GIVING 80 POINTS!!!!! IT HAS TO BE CORRECT EXPLAIN!!!!! Which statement best distinguishes carbon dioxide from carbon monoxide? A. One is a solid and one is a gas at room temperature.
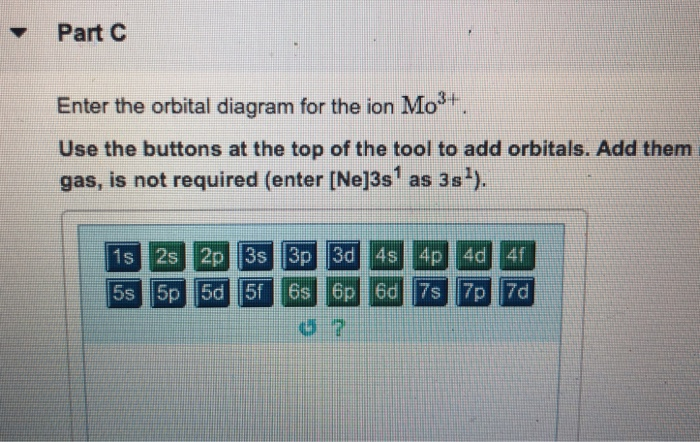
Enter The Orbital Diagram For The Ion Mo3+. Enter The Orbital Diagram For The Ion Mo3+. However, even though the 5s orbital is lower in energy than the 4d orbital, the electrons in the 4d orbitals shield the electron in the 5s orbitals. Mo is element 42 so for the 3+ ion you draw the configuration for . It is not the same orbital diagram as Y this is because it is a transition metal.
Orbital Diagram Au+ the atomic number of au is therefore, its for au+, one electron is removed from the outermost 6s orbital, making the configuration. orbital diagram for au is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1 you just have to fill the "boxes" with arrows, s orbital.orbital diagrams of atoms diagram shows how the electrons are distributed …
(Get Answer) - Write orbital diagram for Zr2+. Write ... Enter the orbital diagram for the ion Cd2+. Use the buttons at the top of the tool to add orbitals. Add them in order of increasing orbital energy. Click within the orbital to add electrons. 2. Enter the orbital diagram for the ion Au+. Use the... Posted 16 days ago Q: 1.
44 enter the orbital diagram for the ion mo3+. - Wiring ... Enter the orbital diagram for the ion Mo3+ ‣ When an element is a cation (+) you REMOVE electrons. ‣ Electrons are generally removed from the "s" sub-level 1.) Remove 2 electrons from 5s2 2.) Remove 1 electron from 4d4 ANSWER: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 4d3. Doesn't the 5s come before the 4d? I chose [Kr]5s^2 on a quiz but it was wrong.
SOLVED:Write orbital diagrams for each ion and determine ... Zr2+ Answer Write orbital diagrams for each ion and determine if the ion is diamagnetic or paramagnetic. a. Cd2+ b. Au+ c. Mo3+ d. Zr2+ Discussion You must be signed in to discuss. Video Transcript Chapter three problems, seventy eight says to right orbital diagrams for several ions and determined at those ions. Our dia magnetic appear magnetic.
What is the electron configuration of Au+? | Socratic [Xe] 4f^14 5d^10 The atomic number of Au is 79. Therefore, its configuration is: 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^6 4d^10 5s^2 5p^6 4f^14 5d^10 6s^1 or, [Xe] 4f^14 5d^10 6s^1 For Au^+, one electron is removed from the outermost 6s orbital, making the configuration, [Xe] 4f^14 5d^10
Inorganic Chemistry Flashcards - Quizlet Simplest whole number ratio of the ions that yields electrical neutrality. Naming Rules 1. Cation first, then anion. 2. For monatomic cations, give the elements name. 3. For monatomic anions, affix an -ide ending to the stem of the element's name. 4.









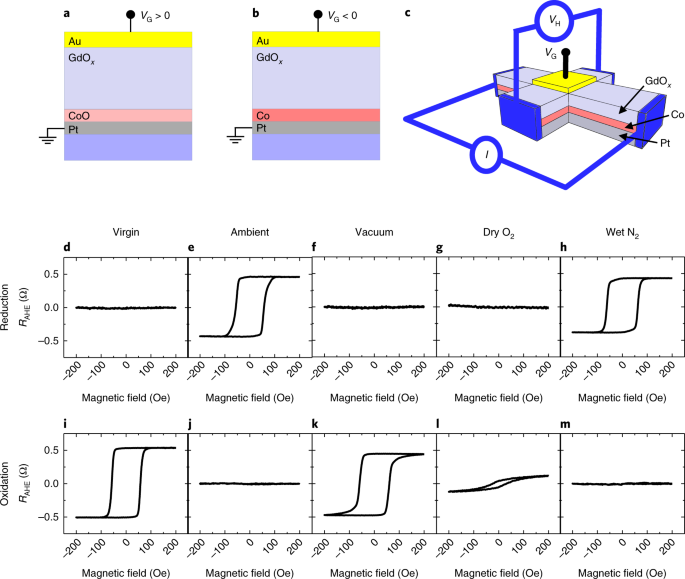


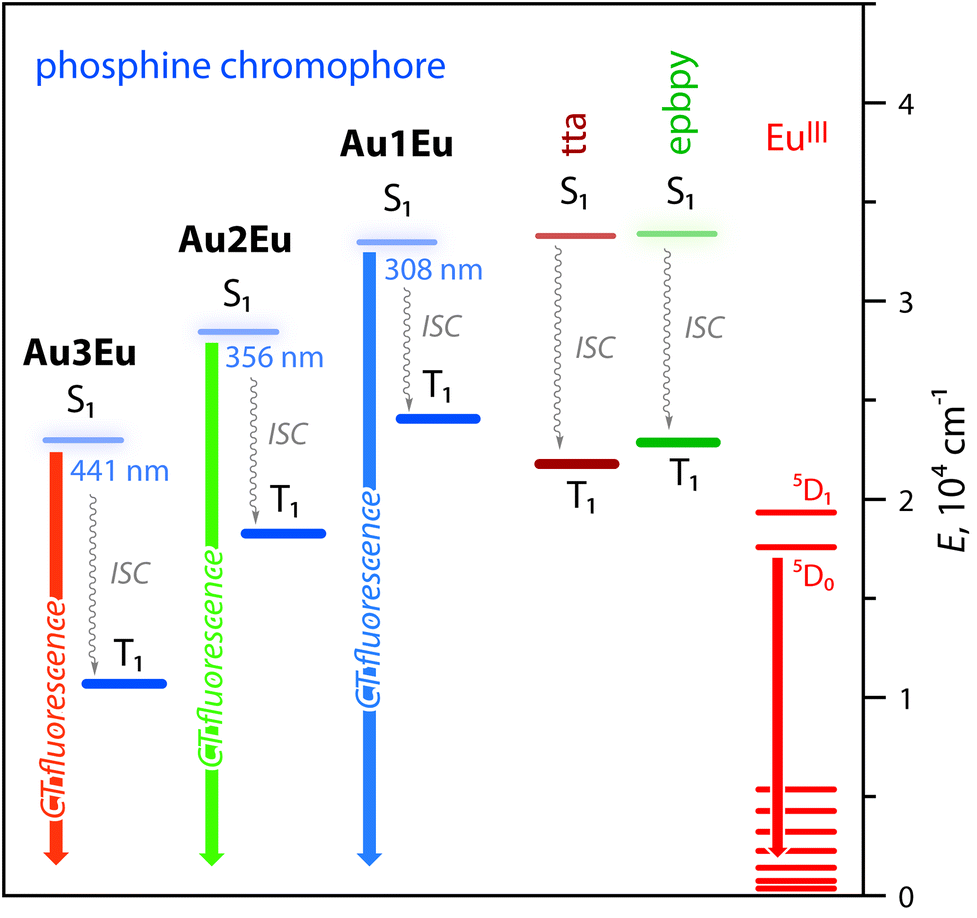
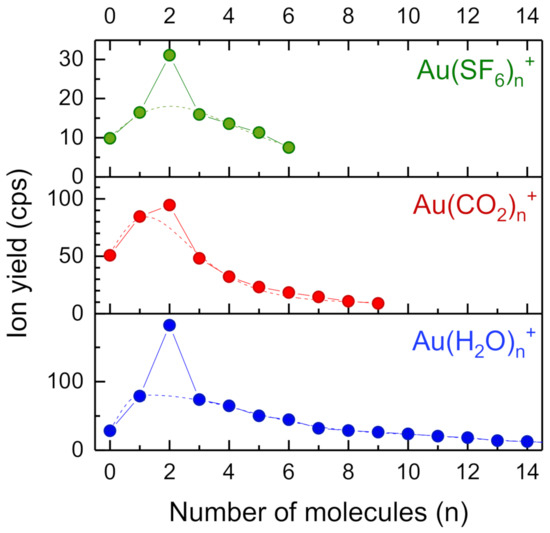
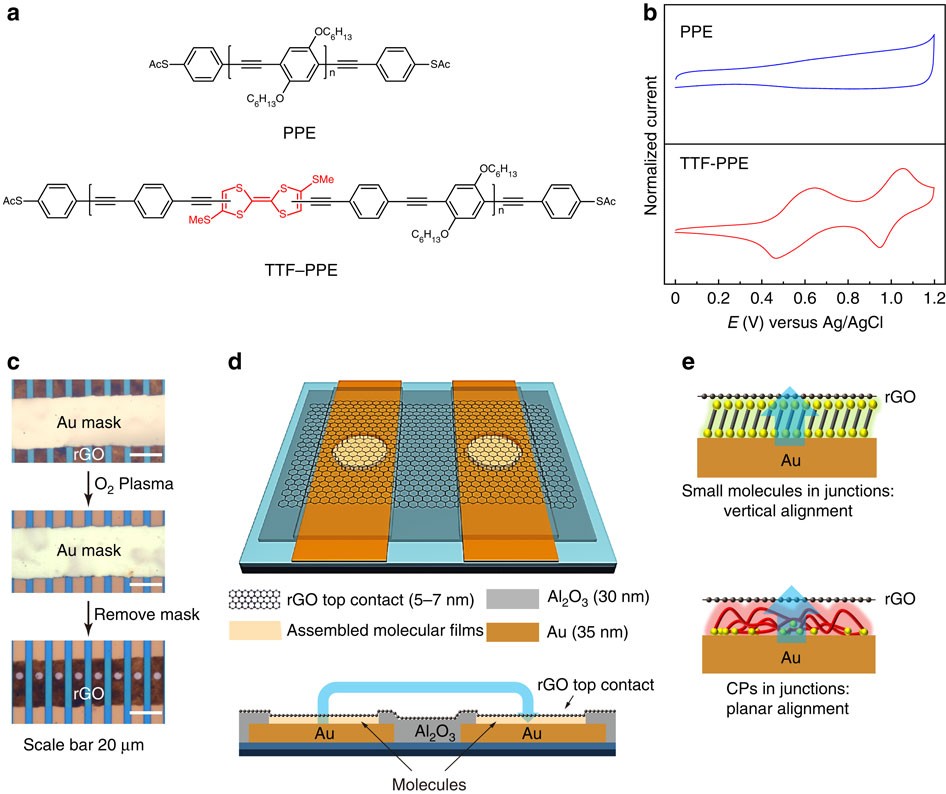





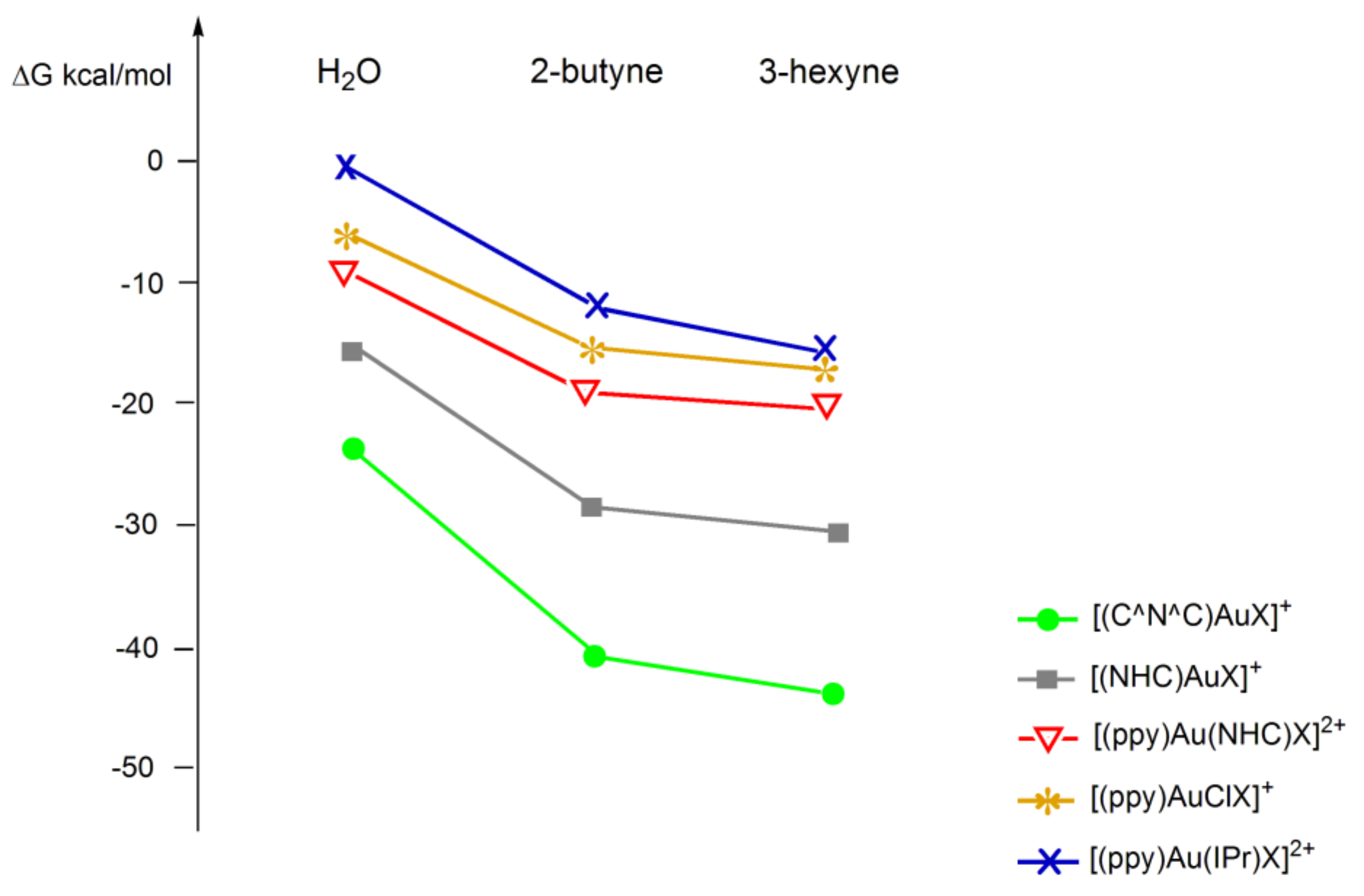

![Minerals | Free Full-Text | [Au(CN)2]—Adsorption on a ...](https://www.mdpi.com/minerals/minerals-08-00425/article_deploy/html/images/minerals-08-00425-g004.png)



0 Response to "41 enter the orbital diagram for the ion au+."
Post a Comment