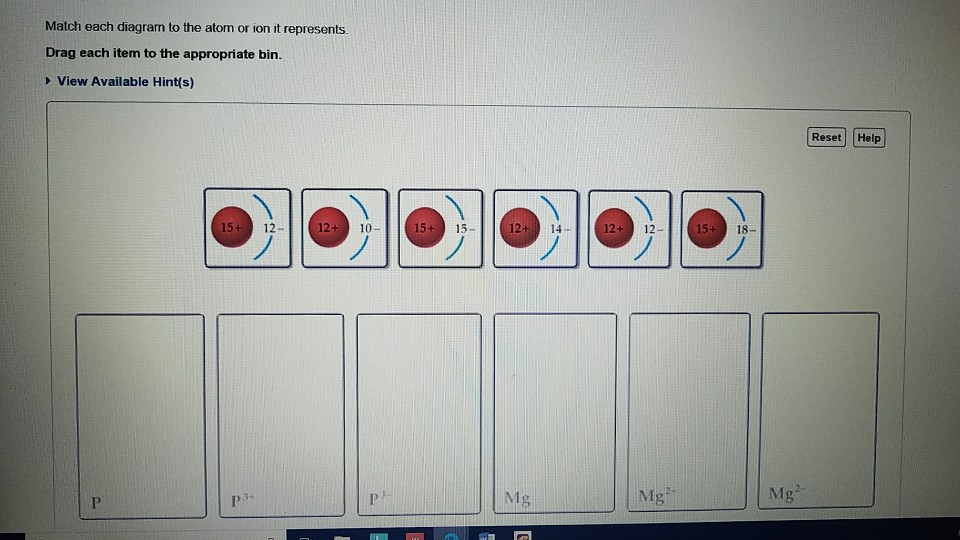
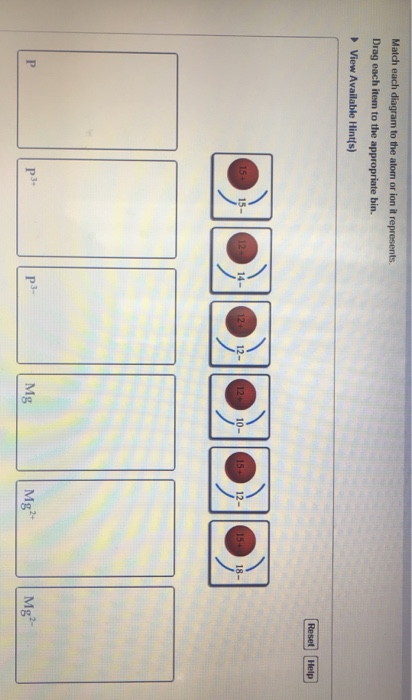
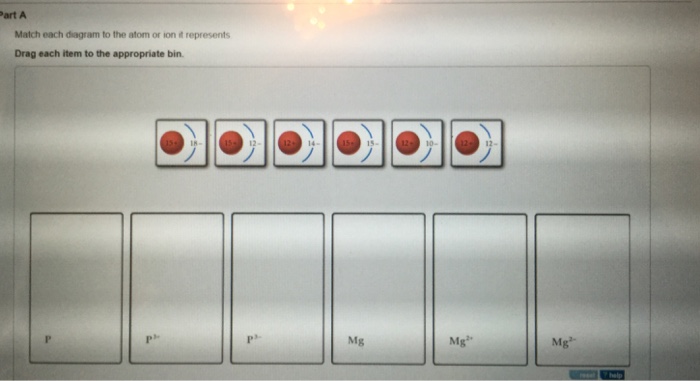
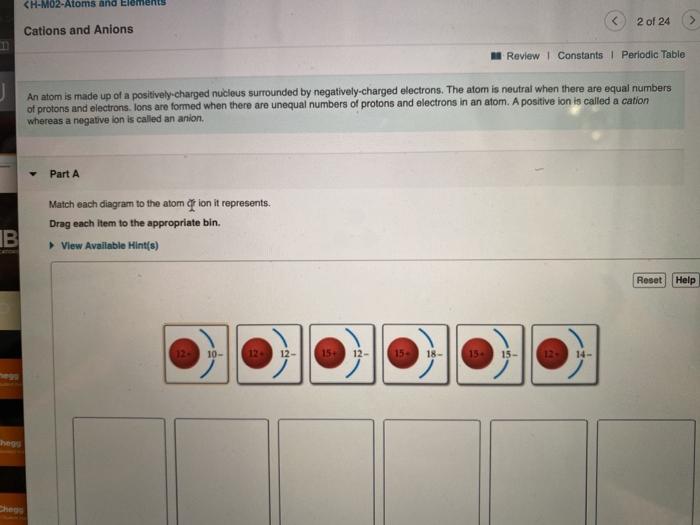
39 match each diagram to the atom or ion it represents.
Atomic Structure and Symbolism - Chemistry An atom that gains one or more electrons will exhibit a negative charge and is called an anion. Positively charged atoms called cations are formed when an atom loses one or more electrons. For example, a neutral sodium atom (Z = 11) has 11 electrons. If this atom loses one electron, it will become a cation with a 1+ charge (11 − 10 = 1+). Atomic Structure and Symbolism | Chemistry for Majors the ion with 54 electrons, 53 protons, and 74 neutrons; the ion with atomic number 15, mass number 31, and a 3− charge; the ion with 24 electrons, 30 neutrons, and a 3+ charge; Write the symbol for each of the following ions: the ion with a 3+ charge, 28 electrons, and a mass number of 71; the ion with 36 electrons, 35 protons, and 45 neutrons
OneClass: Match each diagram to the atom or ion it represents? Match each diagram to the atom or ion it represents? Answer. + 20. Watch. 1. answer. 1.

Match each diagram to the atom or ion it represents.
2. Match each diagram with its correct description ... Match each diagram with its correct description. Diagrams will be used once. . O A B с D E Pure Element - only one type of atom present. Mixture of two elements - two types of uncombined atoms present. Pure compound - only one type of compound present. Mixture of two compounds - two types of compounds present. Mixture of a compound and an element. Part A Match each diagram to the atom or ion it represents ... Part A Match each diagram to the atom or ion it represents. Drag each item to the appropriate bin. You did not open hints for this part. ANSWER: Part B What is the charge on the most stable ion of each of the following elements? Drag each item to the appropriate bin. You did not open hints for this part. Part A Match each diagram to the atom or ion it represents ... Part A PRODUCTS of pyruvate metabolism Match each product of pyruvate metabolism with the condition under which it is produced. Drag each item to the appropriate bin. View Available Hint (s) Reset Help fermentation in human musde aerobic oxidation fermentation in yeast and bacteria lactate ethanol acetyl COA.
Match each diagram to the atom or ion it represents.. Match Each Diagram To The Atom Or Ion It Represents. Drag ... chemical formula and electron dot structures brainmass match each ion with state the number of electrons that must be gained by atoms of each of construct an electron dot diagram for the nitrite ion Part A In the Make Molecules page of the PhET simulation drag the atoms from the labeled bins onto the blank canvas to construct a molecule Learnsmart/Chapter 2 Flashcards - Quizlet 1. An ion with more electrons than its neutral atom is called a(n)-anion. 2. An ion with fewer electrons than its neutral atom is called a(n)-cation. 3. The charge of an ion with more electrons than its neutral atom is-negative. 4. The charge of an ion with fewer electrons than its neutral atom is-positive. 35.png - Match each diagram to the atom or ion it ... View 35.png from CHEM 1017 at University of New Orleans. Match each diagram to the atom or ion it represents. Drag each item to the appropriate bin. View Available What is the Lewis structure of the azide ion? - Chemistry ... Add up how many valence electrons would be needed so that each atom has an octet (for hydrogen: dublet) of its own. Each nitrogen would want eight electrons so: $$3\times8=24\tag{2}$$ Take $(2)-(1)$. This represents the number of electrons the atoms must share, i.e. the number of bonds. $$24-16=8\tag{3}$$ Take $(1)-(3)$. This represents the ...
Match each diagram to the atom or ion it represents. Drag ... Match each diagram to the atom or ion it represents. Drag each item to the appropriate bin. Match each diagram to the atom or ion it represents. Drag each item to the appropriate bin. Solution.pdf. Mastering Chemistry: Chapter 2 Assignment Flashcards - Quizlet Match each element to its period. Drag the appropriate elements to their respective bins. ... Match each diagram to the atom or ion it represents. Drag each item to the appropriate bin. P: 15+ 15-P^3+: 15+ 12-P^3-: 15+ 18- ... Which of the following aspects of the structure of the atom were validated by these experiments? Check all that apply ... Biology Exam Ch1-4 Flashcards - Quizlet 1. The atom shown in the diagram has 4 energy levels or shells. False 2. The atom shown in the diagram above has a total of 5 electron orbitals. True 3. Each energy level consists of 2 or more orbitals. False 4. The second energy level consists of 4 electron orbitals. True 5. 6.4 Electronic Structure of Atoms (Electron Configurations ... The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ).
chapter 7.docx - 1. Which of the following accounts for ... Match each physical parameter of a wave to the correct symbol and units.- ... The magnetic properties of an atom or ion depend on the presence of unpaired electrons. ... Each solution to the equation represents an allowed energy state of the atom and is associated with a particular atomic orbital. PDF Covalent Bonding - simplychemistry.org In the diagram, match the type of model or formula with its representation. ... The formula for a molecular compound represents the atoms that make up one molecule of the compound. The formula for an ionic compound represents one ... from each atom triple three shared electron pairs with three electrons Chem: Chapter 2 (Atoms, Molecules, and Ions ... - Quizlet Chem: Chapter 2 (Atoms, Molecules, and Ions) Practice questions. Write the chemical formula of the ionic compound that is composed of Ti^4+ and O^-2 ions. *the formula of an ionic compound is written as an empirical formula. Subscripts must always be reduced to the smallest ratios. To represent an ion, the charge of the ion is written as a ... The Lewis Electron-Dot Symbols of Elements - STLCC.edu The Lewis Electron-Dot Symbols of Elements. Gilbert N Lewis is widely known for his use of simple symbolic representations of elements that show valence electrons as dots. You've seen the Bohr's diagram for the first 18 elements. Sometimes it is more convenient to represent the elements by its Lewis electron dot symbol.
Solved Match each diagram to the atom or ion it represents ... Question: Match each diagram to the atom or ion it represents Drag each item to the appropriate bin. This problem has been solved! See the answer See the answer See the answer done loading
O CHEM Exam 1 (Tophat) Flashcards - Quizlet Which of the following diagrams correctly represents the hybridization of the oxygen atom in the acylium ion? Note that the oxygen has a positive charge. Instead of 6 valence electrons it has 5. ... Match each structure to the correct name. 1) 4-ethyl-2¸3-dimethylhexane 2) 4-(tert-butyl)-3-methylheptane ...
Atom Diagrams: Electron Configurations of the Elements For each electron shell atom diagram, the element symbol is listed in the nucleus. The electron shells are shown, moving outward from the nucleus. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The element atomic number and name are listed in the upper left.
PDF Your Name: Your ID: Question The atom or ion has 1 core electrons and 2 valence electrons. 1. 2. ... Match each Lewis structure with letter associated with the description. Each letter is used only once. ... Choose the diagram that represents the ground state configuration of the neutral nitrogen atom.
Atoms, Isotopes, Ions, and Molecules | Boundless Biology Each electron has a negative charge (-1) equal to the positive charge of a proton (+1). Neutrons are uncharged particles found within the nucleus. Key Terms. atom: The smallest possible amount of matter which still retains its identity as a chemical element, consisting of a nucleus surrounded by electrons.
Solved Match each diagram to the atom or ion it represents ... Chemistry questions and answers. Match each diagram to the atom or ion it represents. Drag each item to the appropriate bin View Available Hint (s) Reset Help 15+ 12- 12+10- 15+15- 1214 12+ 12- 1518- 2- Mg Mg Mg. Question: Match each diagram to the atom or ion it represents.
Drag the elements into the appropriate bins ... - Course Hero The atom is neutral when there are equal numbers of protons and electrons. Ions are formed when there are unequal numbers of protons and electrons in an atom. A positive ion is called a cation whereas a negative ion is called an anion. Part A Match each diagram to the atom or ion it represents. Drag each item to the appropriate bin.
NCERT Exemplar Class 9 Science Solutions Chapter 4 ... NCERT Exemplar Solutions Class 9 Science Chapter 4 - Free PDF Download. NCERT Exemplar Solutions for Class 9 Science Chapter 4 Structure of Atom benefits the students by giving them extra knowledge on the concepts involved in CBSE Class 9 Structure of Atom. It is advised for the students to get acquainted with this NCERT Exemplar in order to lay a strong foundation for your future studies.
Part A Match each diagram to the atom or ion it represents ... Part A PRODUCTS of pyruvate metabolism Match each product of pyruvate metabolism with the condition under which it is produced. Drag each item to the appropriate bin. View Available Hint (s) Reset Help fermentation in human musde aerobic oxidation fermentation in yeast and bacteria lactate ethanol acetyl COA.
Part A Match each diagram to the atom or ion it represents ... Part A Match each diagram to the atom or ion it represents. Drag each item to the appropriate bin. You did not open hints for this part. ANSWER: Part B What is the charge on the most stable ion of each of the following elements? Drag each item to the appropriate bin. You did not open hints for this part.
2. Match each diagram with its correct description ... Match each diagram with its correct description. Diagrams will be used once. . O A B с D E Pure Element - only one type of atom present. Mixture of two elements - two types of uncombined atoms present. Pure compound - only one type of compound present. Mixture of two compounds - two types of compounds present. Mixture of a compound and an element.



















![PDF] Modeling the Behavior of Inclusions in Plastic ...](https://d3i71xaburhd42.cloudfront.net/f23e8744f2c98fd01668f04b4b32297e21a450a7/30-Figure17-1.png)


0 Response to "39 match each diagram to the atom or ion it represents."
Post a Comment