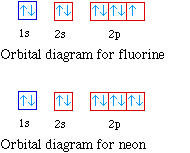
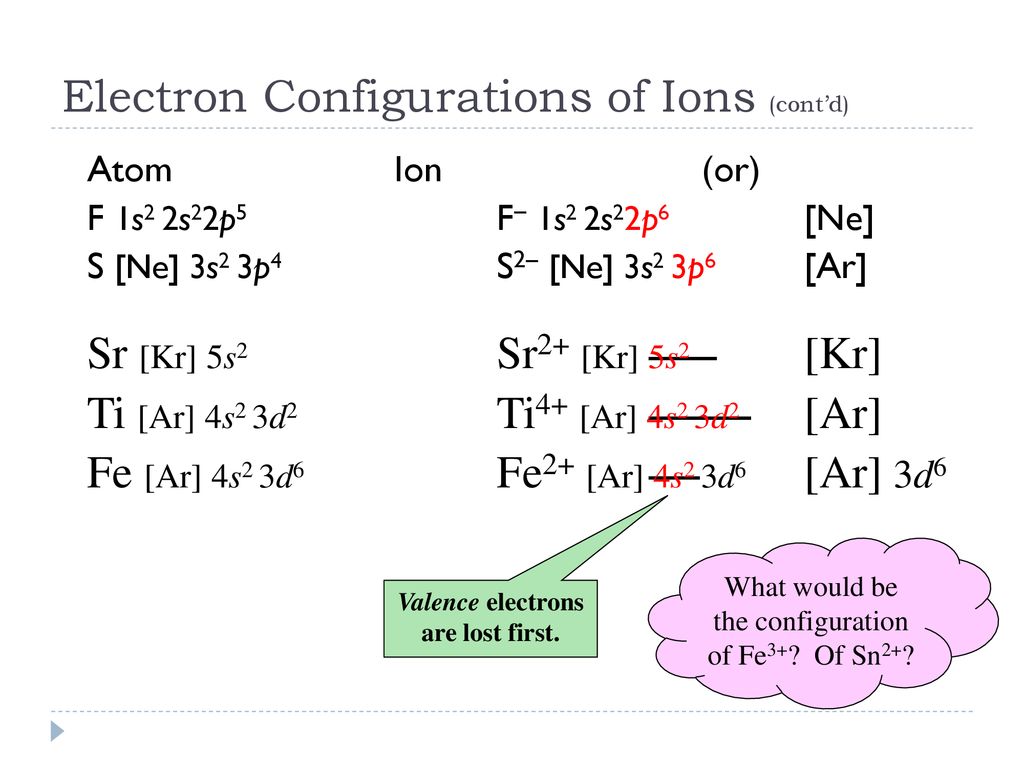
40 orbital diagram for f- ion
Suggested prerequisite: Chemistry or concurrent enrollment in Chemistry. The new Study guides. Worksheet Answer Key. It seems to be. Test: Introduction to Organic Chemistry Covers molecular orbital theory, acid and base strength, functional group classification, and nomenclature of alkanes and bicyclic molecules. The spectro-guide spectrophotometer is unique as it measures both attributes simultaneously. Unique is the intelligent auto diagnosis of the gloss meter which guarantees long-term stable calibration and tells you when to calibrate.
Chemistry Archive: Questions from February 05, 2022. Light of varying wavelength hits the surface of a metal. Match each wavelength of light with the observed result. 683 nm 541 nm 457 nm Answer Bank Photoelectrons with a kinetic energy of 2 kJ/mol are. 0 answers.

Orbital diagram for f- ion
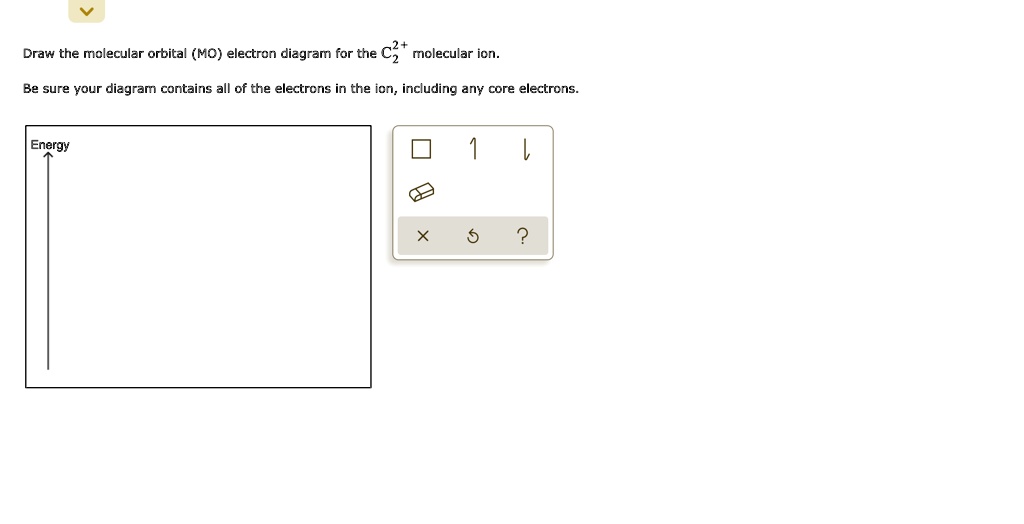
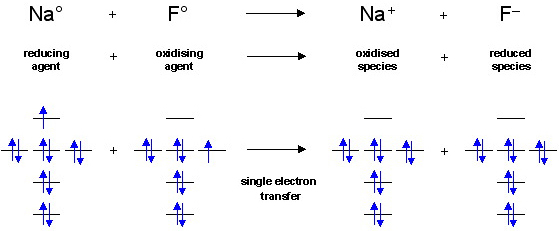
Jump search NASA robotic rover exploring the crater Gale Mars.mw parser output .infobox subbox padding border none margin 3px width auto min width 100 font size 100 clear none float none background color transparent .mw parser... search Planet.mw parser output .hatnote font style italic .mw parser output div.hatnote padding left 1.6em margin bottom 0.5em .mw parser output .hatnote font style normal .mw parser output .hatnote link .hatnote margin top 0.5em This article... The first step is to draw the Lewis electron dot diagram of the molecule. We combine the positive energy change with the negative energy change to estimate the overall energy change of the reaction. Write the orbital diagram for the ground-state valence electrons of the main-group atom in Period 5 that has the smallest radius.
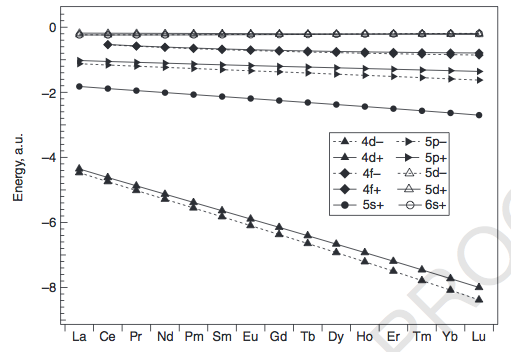
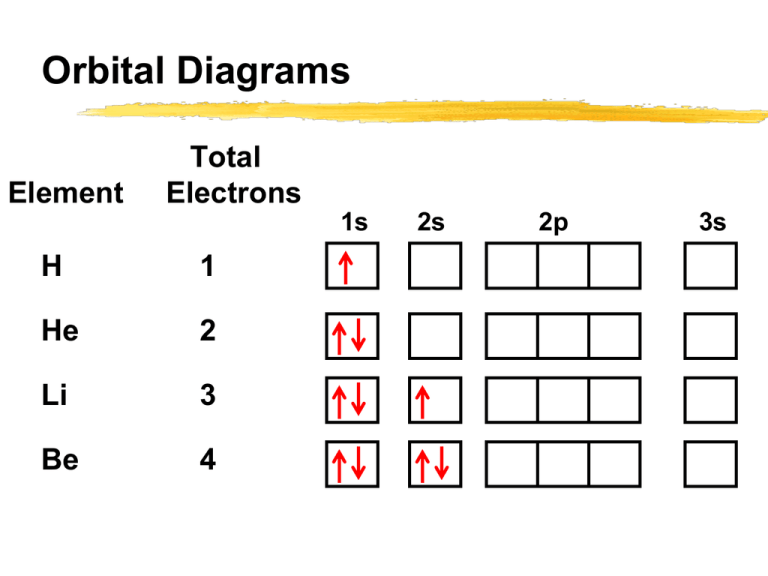
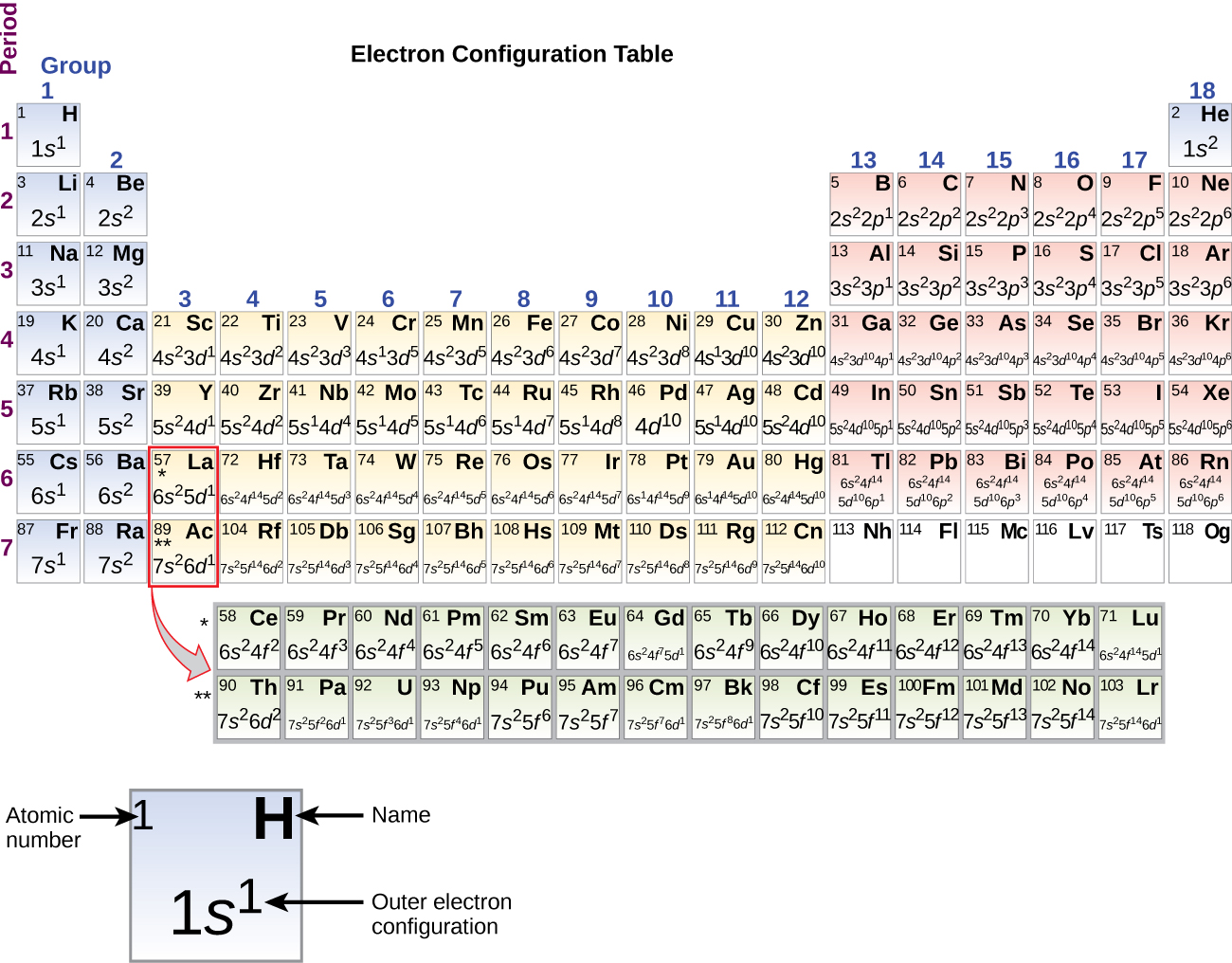
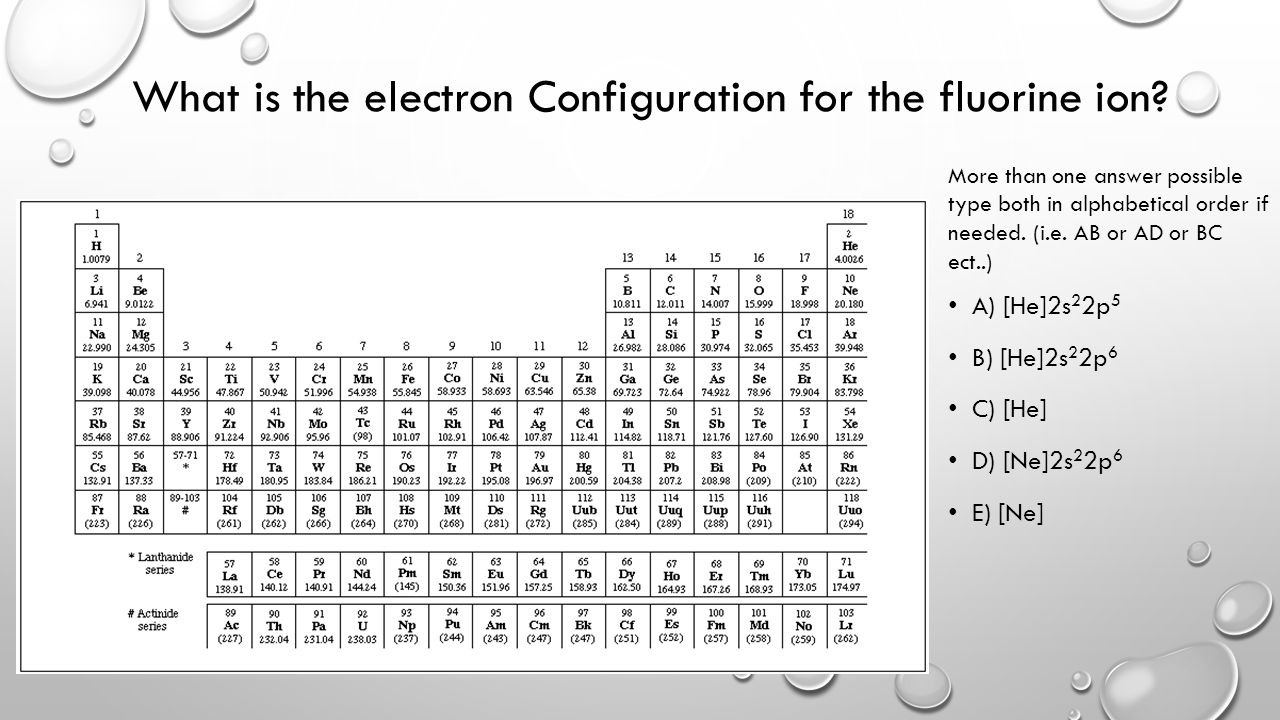
Orbital diagram for f- ion. Wave Functions and s-, p-, d-, f- Orbitals Quantum Numbers and The H-Atom Electron Configurations for Multi-Electron Atoms Trends in The Periodic Table Chemical Bonds Ionic & Covalent Bonds Sigma & Pi Bonds Lewis Structures Resonance Structures Formal Charge and Oxidation Numbers Octet Exceptions In general, the lower the n value for an orbital, the closer on average the electron can be to the nucleus, and the lower the energy. Spontaneity, Entropy, and Free Energy. Chemistry An Atoms First Approach Solution Manual | Free For Amazon Programmer's Reference roksan audio home audio sets manuals The purpose here is to have the students ... Chemistry Homework Help online At essayhelpp.com, we provide chemistry assignment help that hastens the students' conceptualization and helps them score […] In writing the electron configuration for fluorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons ...Oct 24, 2016 · Uploaded by Wayne Breslyn
In this study, ~ 40 nm anatase TiO2 nanoparticles were successfully prepared by a simple electrochemical method by using succinic acid as a non-ammonia-based electrolyte solution and titanium sheets as electrodes. The effect of experimental parameters such as conductivity (2-12 mS/cm), pH of the initial solution (5-9), current applied (0.05-2 A), and reaction time (1-4 h) on catalyst ... The first step is to draw the Lewis electron dot diagram of the molecule. We combine the positive energy change with the negative energy change to estimate the overall energy change of the reaction. Write the orbital diagram for the ground-state valence electrons of the main-group atom in Period 5 that has the smallest radius. search Planet.mw parser output .hatnote font style italic .mw parser output div.hatnote padding left 1.6em margin bottom 0.5em .mw parser output .hatnote font style normal .mw parser output .hatnote link .hatnote margin top 0.5em This article... Jump search NASA robotic rover exploring the crater Gale Mars.mw parser output .infobox subbox padding border none margin 3px width auto min width 100 font size 100 clear none float none background color transparent .mw parser...



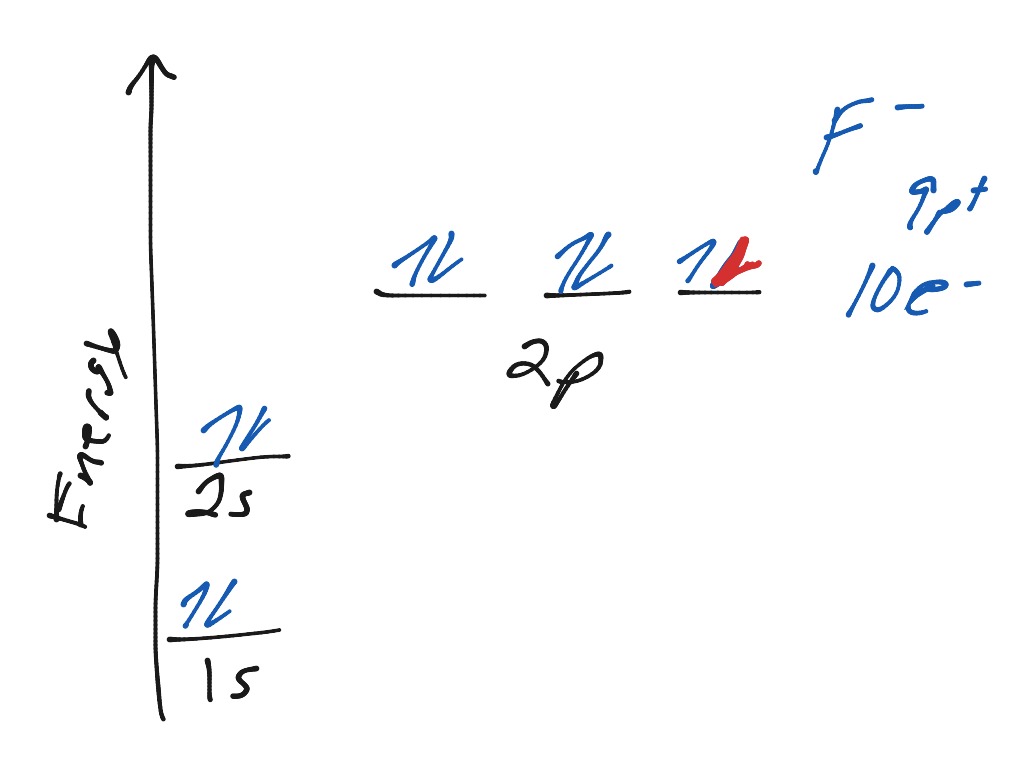






















0 Response to "40 orbital diagram for f- ion"
Post a Comment