39 nf molecular orbital diagram
(6 − 0) = B.O. 2 Looking at the molecular orbital diagram, you can see that there are no ... Problem 3 Consider the molecule NF and the ions NF+ and NF- . https://i.imgur.com/GgRlFtK.jpg (Not homework) I am trying to improve by using past papers. Can someone explain how to solve these 3 questions?
What happens to the molecular orbital diagram when a metal-ligand complex is oxidized? Oxidation removes an electron, e.g. you go from d8 metal to d7 metal. As consequence the antibonding orbital has an unpaired electron making the complex less stable (weaker M-L bond, since less pi-backdonation), but how does it change the gap between the metal MO and LUMO of ligand, as well as the gap between the metal MO and HOMO of the ligand?
Nf molecular orbital diagram
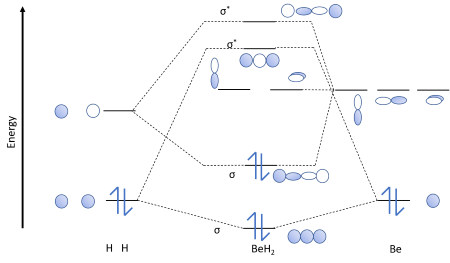
Shouldn’t you count the valence electrons for Be (which is 2) and subtract 1 because of the + sign? For O2, N2, NO, F2, etc, you count the number of valence electrons instead of the atomic number. Why is it that for Be, though, you look at the atomic number instead of the number of valence electrons it has? I apologize if this is a stupid question, but I appreciate any clarification on this Next: · Molecular orbitals water · MO Diagram CO2 · Lewis Diagrams Made Easy: How to Draw Lewis Dot Structures · General Chemistry 1 Review Study ... I’m a little confused on the connection between a molecules molecular orbital diagram and it’s individual atomic hybridization. Can anyone help me? Thank you
Nf molecular orbital diagram. Bonding and antibonding orbitals are illustrated in MO diagrams, and are useful for predicting the strength and existence of chemical bonds. hey! I have a question: how do i draw a molecular orbital diagram for SO2? i only found examples for diatomic diagrams and im not sure how to do it if i have more then two atoms in the molecule. I’ve been tasked with drawing rhe MO diagram for Sulfure Oxide and I’m not sure about the energies of the relatove orbitals. Since Oxygen is more electronegative I expect the 2s and 2p orbitals to have much lower energy than the 3s and 3p orbitals sulfur has. But the energy difference would be really high then. So I’m not sure what 2 orbitals combine to form the sigma 3s or sigma* 3s orbital. The difference in energy kevels confuses me as every example I’ve done has the same orbitals (2s,2p’s) c... I've been getting the hang of creating MO diagrams and I understand the very basics. My problem is in the 2p orbital's bonding section where sometimes the pi 2p section is lower energy than the sigma 2p section (i.e MO diagram for B2 diatomic molecule). I understand that the lower energy must be filled in first and so my question is, how do I know if the pi 2p is lower energy than sigma 2p?
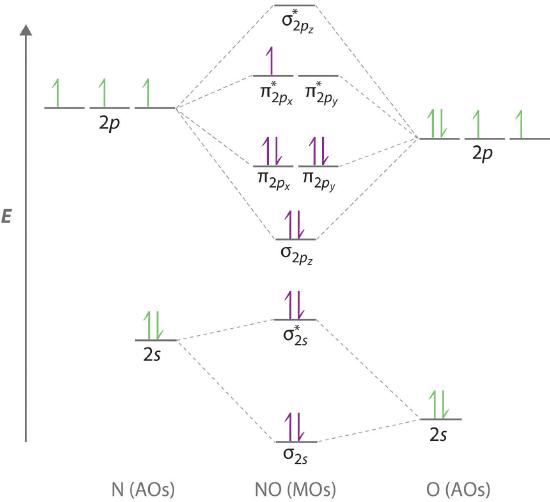
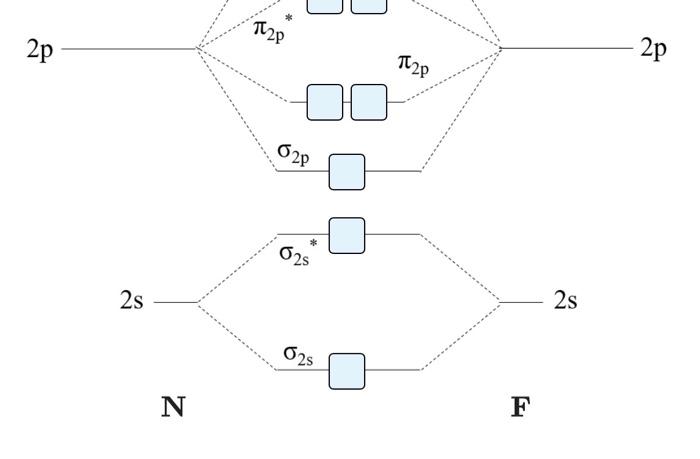
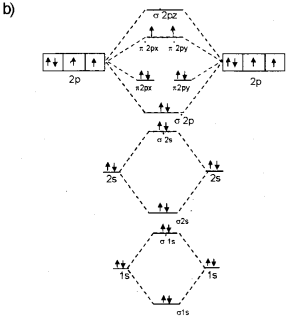
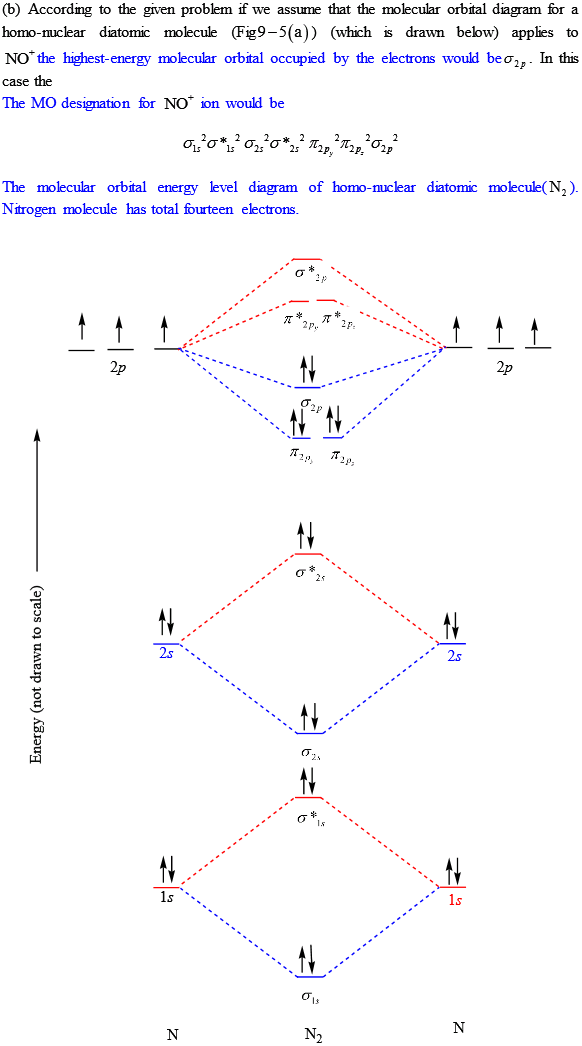
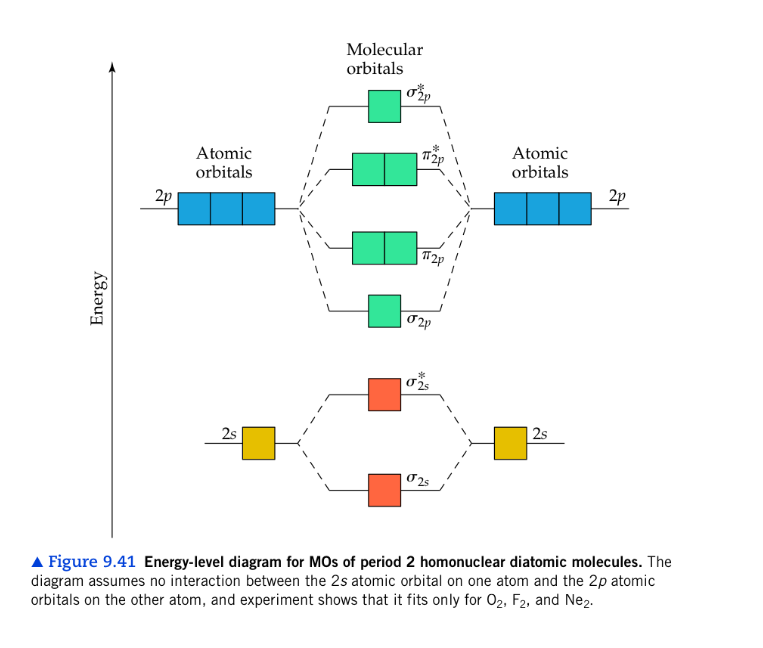
Transcribed image text: a) Complete the molecular orbital diagram for NF, nitrogen monofluoride below. Electrons can be added to the orbitals by clicking ... 05.11.2015 ... Lavelle said that whenever we have a heteronuclear molecule, if one or both atom(s) has Z<8, then we would use the diagram where the pi px and ... Hi I am a PhD candidate and I need help making a frontier molecular orbital visualization diagram for the molecule pentacene. I only need 4 molecular orbital visualizations and they are HOMO, LUMO, HOMO-1, and LUMO +1. I am currently using chemissian with the imput from IQMOl but I am having difficulties getting a visual MO composition output. Can anyone help me? (b) Construct a molecular orbital energy diagram for NF. For this drawing consider both the s and p valence orbitals on each atom, but do not include orbital ...
molecular orbitals in the diagram suggest a double bond. c. The σ2s, σ2s. *, σ2p, and σ2p. * orbitals exhibit C∞v symmetry, with the. NF bond axis the ... I need to construct the molecular orbital diagram for the hypothetical species Li4, which has the following geometrical arrangement: https://preview.redd.it/npsjre5pch571.png?width=197&format=png&auto=webp&s=c2a7948c2efa04a975bee1db722838fae7482456 The first step is to identify the point symmetry group. In this particular case, we consider that there is only one axis of rotation of order four (actually, other symmetry elements can be observed, but this is a previous consi... For each of the two species NF and NF+, (a) draw MO energy level diagrams; (b) write out electron configurations; (c) determine bond orders and predict ... 4.* (1997 1 7) Consider the diatomic molecule NF. Draw its molecular orbital energy diagram. A. Using the LCAO-MO scheme ...
I didn't take Chem 2070 (used AP credit), so I know essentially nothing about molecular orbitals. However, Professor Fors said we would see molecular orbitals quite a bit in 3580 and I already don't understand the first lecture (HOMO and LUMO who?). How in-depth with molecular orbitals does the class go, and is understanding MOs actually important for the exams or is it more just background information to help explain reactions, but you don't really need to know it?
Hi! I am a physicist student preparing a solid state physics exam and we discussed molecular orbitals, (sigma, pi) bonding, hybridization, etc... way more than I would like. Since it is the first time I see it some books would be appreciated!
Answer to: NF is a known molecule! Construct a molecular orbital diagram for NF, an include sketches showing how the valence orbitals of N and F...
4 Draw the valence molecular orbital diagram for NF State the bond order the from NH 4 at University of California, San Diego.
I’m a little confused on the connection between a molecules molecular orbital diagram and it’s individual atomic hybridization. Can anyone help me? Thank you
Next: · Molecular orbitals water · MO Diagram CO2 · Lewis Diagrams Made Easy: How to Draw Lewis Dot Structures · General Chemistry 1 Review Study ...
Shouldn’t you count the valence electrons for Be (which is 2) and subtract 1 because of the + sign? For O2, N2, NO, F2, etc, you count the number of valence electrons instead of the atomic number. Why is it that for Be, though, you look at the atomic number instead of the number of valence electrons it has? I apologize if this is a stupid question, but I appreciate any clarification on this
























0 Response to "39 nf molecular orbital diagram"
Post a Comment