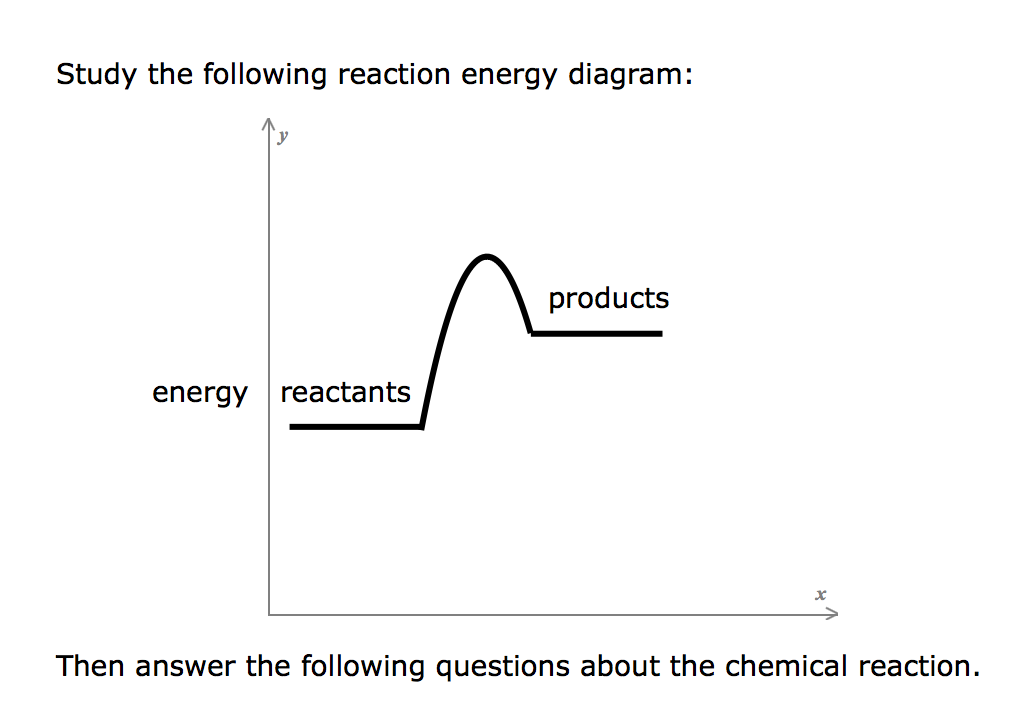
40 study the following reaction energy diagram:
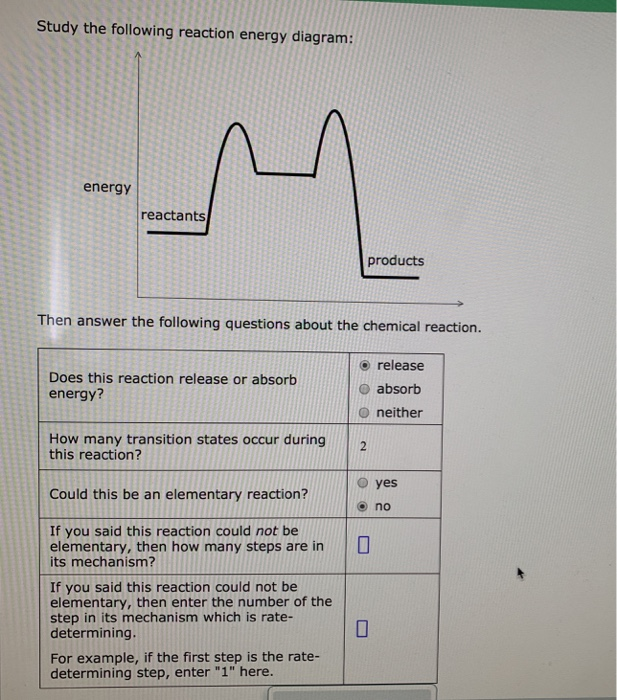
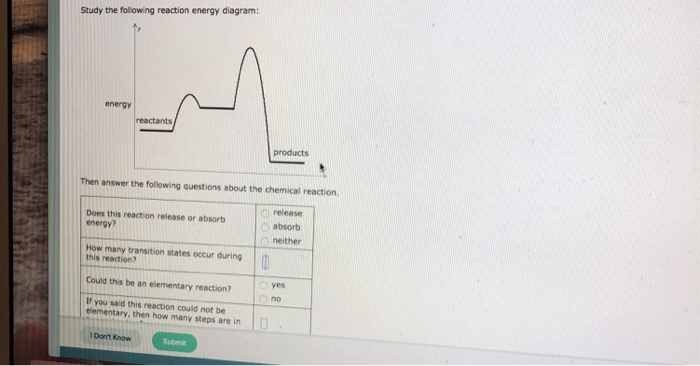
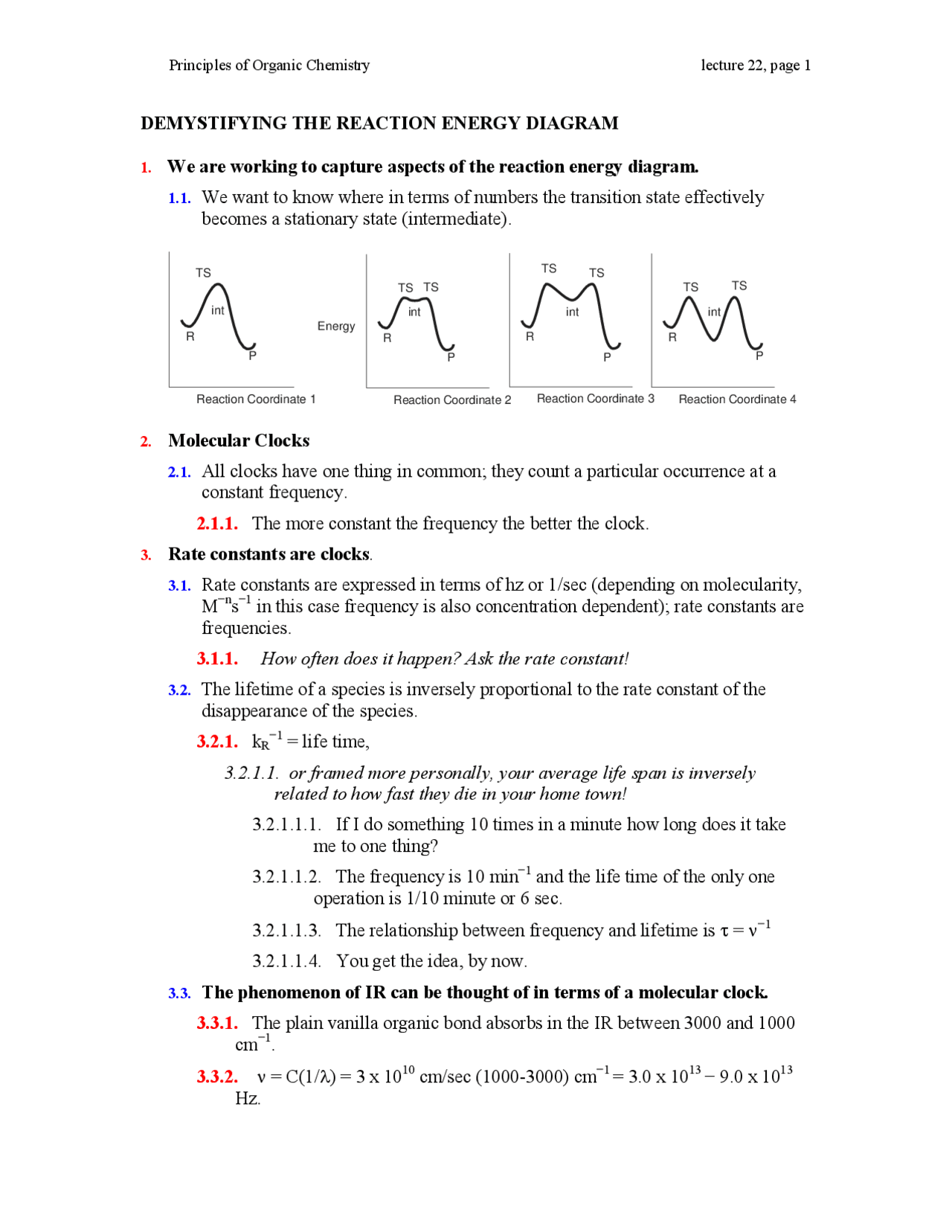
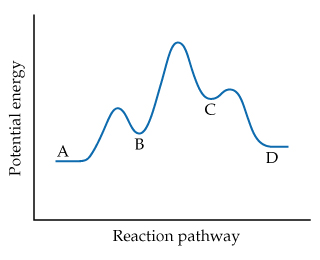
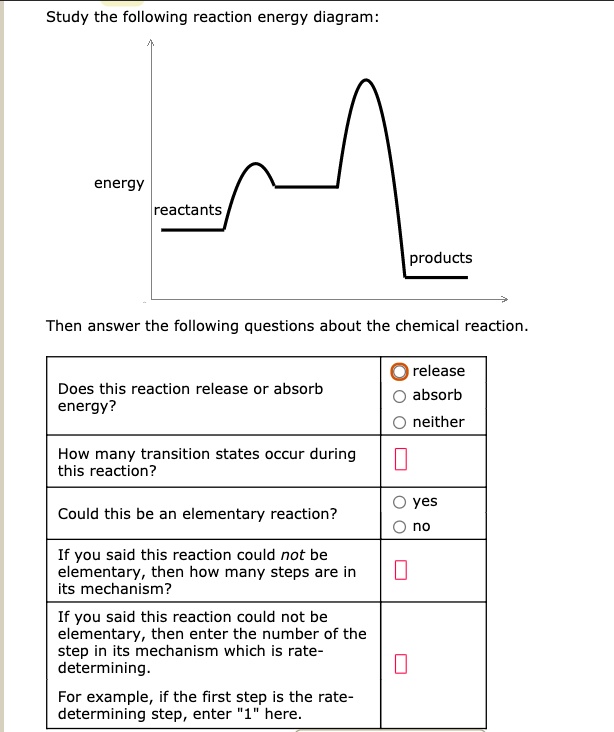
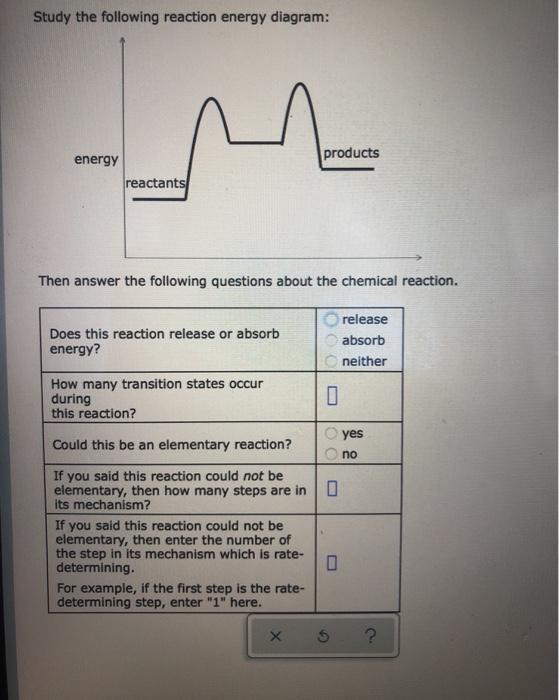
Study the following reaction energy diagram: energy reactants, products Then answer the following questions about the chemical reaction. release Does this reaction release or absorb O absorb energy? O neither How many transition states occur during this reaction? O yes Could this be an elementary reaction? Consider the following diagrams which show the progress for the reaction A (blue) ⇌ B (red). The equilibrium constant (K) for this reaction is 0.8. At which point does the reaction reach equilibrium? The equilibrium constant is the products divided by the reactants. A K value of 0.8 is consistent with image choice C where the ratio of product ...
Which of the following statements correctly describe the key aspects of drawing a reaction energy diagram. For an exothermic reaction the energy of the products is less than the energy of the reactants.
Study the following reaction energy diagram:
Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams. Here the energy of the reactant is less than the product i.e ; it is an endothermic reaction. Hence, in this reaction the energy is absorbed. Her …. View the full answer. Transcribed image text: Study the following reaction energy diagram: Does this reaction release or absorb energy? release absorb neither yes no 0 How many transition states ... Using Reaction Diagrams to Compare Catalyzed Reactions The two reaction diagrams here represent the same reaction: one without a catalyst and one with a catalyst. Identify which diagram suggests the presence of a catalyst, and determine the activation energy for the catalyzed reaction: Solution
Study the following reaction energy diagram:. 34 draw an energy diagram for the following reaction. This preview shows page 6 - 8 out of 8 pages. 1. Show the mechanism for the following elimination, assuming that it is a concerted reaction (E2). I OMe 2. Show the mechanism for the following elimination, assuming that it is a stepwise reaction (E1). Cl OMe 3. The study of energy changes (particularly heat) in chemical reactions is known as chemical thermodynamics. This is also sometimes called thermochemistry. ... When a chemical reaction occurs, bonds in the reactants break, while new bonds form in the product. The following example explains this. Ask any question and get an answer from our subject experts in as little as 2 hours. Study the following reaction energy diagram: n products energy reactants Then answer the following questions about the chemical reaction.
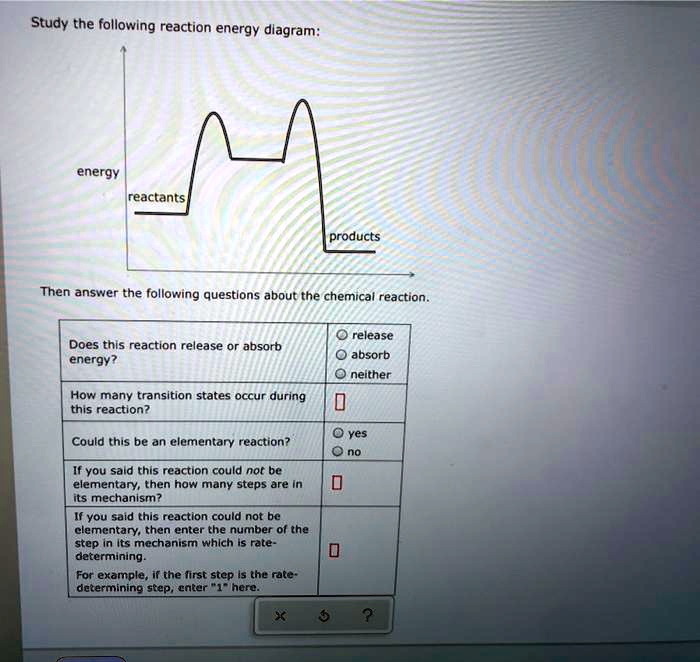
Answer to Consider the following reaction energy diagram: 800 a) How many elementary steps are in the reaction mechanism? b) Deter... The reaction NO2 (g) + CO (g) → NO (g) + CO2 (g) has ΔH°overall = -226 kJ/mol. A proposed reaction mechanism is shown. Choose the statement (s) that accurately describe the reaction energy diagram for the above reaction. -There will be three peaks. -The Ea of the first step will be larger than the second or third step. Ask any question and get an answer from our subject experts in as little as 2 hours. Explanation: The fully filled in reaction coordinate diagram is displayed below. The arrow marked in the question represents the activation energy, which is the energy barrier that must be overcome in order for the reactants to form products. This reaction is also exothermic because the energy of the products is lower than that of the reactants.
It decreases the kinetic energy of reactants, enabling them to undergo chemical change more easily. Correct Answer: By lowering the activation energy, an enzyme increases the rate of the reaction. Review B. 4. Consider the following energy diagram for a chemical reaction. A reaction coordinate diagram is a graph that plots energy versus reaction progress. The amount of energy that needs to be added is called the activation energy, which is the point where the line ... Click here to get an answer to your question ✍️ Which one of the following reaction energy diagrams best repre ollowing reaction energy diagrams best ... August 22, 2018 - Get the detailed answer: 95. Consider this energy diagram: Energy -Reaction progress- a. How many elementary steps are involved in this reaction? b. Label
Ask any question and get an answer from our subject experts in as little as 2 hours.
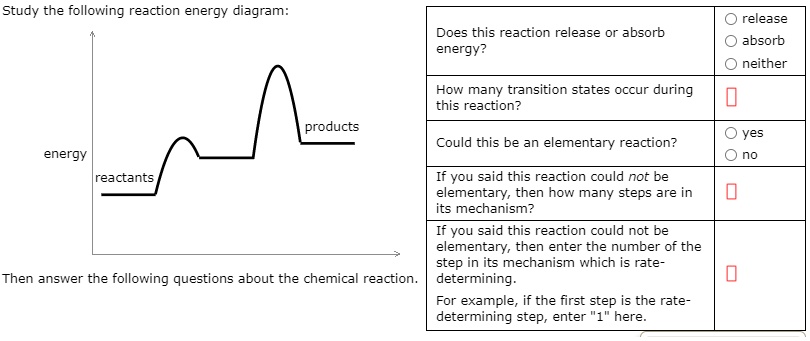
Transcribed image text: Study the following reaction energy diagram: products energy reactants Then answer the following questions about the chemical reaction Does this reaction release or absorb energy? release o absorlb o neither How many transition states occur during this reaction? yes Could this be an elementary reaction? O no If you said this reaction could not be elementary, then how ...
16.4g Deducing information about reaction mechanisms from a reaction energy diagram. Watch later. Share. Copy link.
Ask any question and get an answer from our subject experts in as little as 2 hours.
Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.
Transcribed image text: Study the following reaction energy diagram: energy reactants products Then answer the following questions about the chemical reaction. Does this reaction release or absorb energy? release absorb neither How many transition states occur during this reaction? 0 yes Could this be an elementary reaction? no If you said this reaction could not be elementary, then how many ...
Q. The diagram shows energy changes in a specific chemical reaction with and without the addition of an enzyme to the reaction. Which of the following questions can be answered by the diagram? ... Q. To study the effects of catalase on its substrate, hydrogen peroxide, students made solutions of different hydrogen peroxide concentrations. ...
The energy change in the water bath will be the _____ magnitude in energy as the chemical reaction or phase change, just with the _____. If the water bath gains energy, its temperature goes _____, meaning the energy of the chemical reaction or phase change went down (it lost energy) and vice versa.
Study the following reaction energy diagram: products energy reactants Then answer the following questions about the chemical reaction.
Which of the following statements is not true? A) Two reactions can have identical values for DH° but very different Ea values. B) The larger the activation energy, the slower the reaction. C) DH° determines the height of the energy barrier. D) The lower the activation energy, the faster the reaction.
Request unsuccessful. Incapsula incident ID: 875000140237035127-630220037666046861
1. Base your answers to the following questions on the energy diagram below. Legend — = uncatatyzed pathway — --- = Cu-catalyzed pathway reaction coordinate a. Does the addition of copper change the rate of reaction? Explain. b. How would increasing the temperature of the uncatalyzed pathway affect the reaction? 6.
This meiosis study guide introduces you to the basics of meiosis and the meiotic process. Sex cells are produced through this two stage process. TIM VERNON / SCIENCE PHOTO LIBRARY / Getty Images Meiosis is a two-part cell division process i...
in this problem of nuclear physics, we have to find the energy relate in the reactions. First reaction is this is helium four plus helium four is converted ...
Study the following reaction energy diagram: products energy reactants Then answer the following questions about the chemical reaction: release absorb ...
A reaction is represented by the following reaction energy diagram. How many elementary steps are present in the reaction?
A small US study has found a link between consumption of 'energy drinks' and high blood pressure or heart disease risk. A small US study has found a link between consumption of “energy drinks” and high blood pressure or heart disease risk. ...
Answer to Consider the following reaction energy diagram: How many elementary steps are in the reaction mechanism? 1 2 3 4 Which s...
Transcribed image text: study the following reaction energy diagram: products energy Then answer the following questions about the chemical reaction. release Does this reaction release or absorb absorb energy? neither How many transition states occur during this reaction? Could this be an elementary reaction? said this reaction could not be elementary, then how many steps are in its mechanism?
Answer the following questions regarding the kinetics of chemical reactions. (a) The diagram below at right shows the energy pathway for the reaction O3 + NO NO2 + O2. Clearly label the following directly on the diagram. (i) The activation energy (Ea) for the forward reaction (ii) The enthalpy change ( H) for the reaction
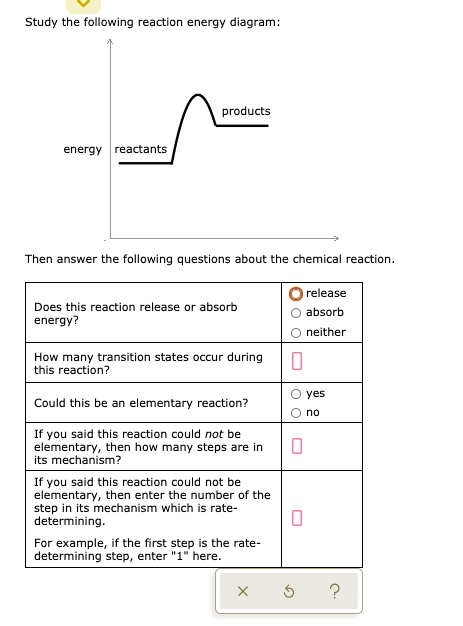
95% (19 ratings) 1. The products have higher energy than the reactants. So, the reaction absorbs energ …. View the full answer. Transcribed image text: Study the following reaction energy diagram: products energy reactants Then answer the following questions about the chemical reaction.
Draw the potential energy diagram of an exothermic reaction. Illustrate, using two-way arrows, the items below. Give a title to each of the axes and to the diagram, and indicates the energy level of the components of the reaction. The change in enthalpy The activation energy of the direct reaction The energy of the reverse reaction.
1i. Draw an energy vs reaction coordinate diagram to illustrate a reaction in which the energy of the products is greater than the energy of the reactants. Label all quantities as per Fig. 1. See diagram (3) in sample exercise 14.10 on pg 595 of Brown and LeMay, 11th ed.
100% (11 ratings) Part A :- As the reactant are at higher energy level than products, this means the energy is being released in the reaction. Answer part A …. View the full answer. Transcribed image text: Study the following reaction energy diagram: energy reactants products Then answer the following questions about the chemical reaction ...
Step-by-step explanation. Question (3 ) solution ( a) Energy diagram for Exothermic with high Eact is as shown below .. Activation energy potential energy Energy energy Released. of Reactant energy of product Progress of Reaction - Energy Released in Exothermic Reaction. ( b) Energy diagram for Endothermic with low Eact is as shown below .
Electrochemistry—the study of the interchange of chemical and electrical energy ... Calculate the cell voltage for the following reaction. Draw a diagram of the galvanic cell for the reaction and label completely. Fe3+ (aq) + Cu(s) ... E = Energy produced by reaction T = Temperature in Kelvins
12. Consider the following potential energy diagram that represents two different reactions. Which of the following statements is correct?
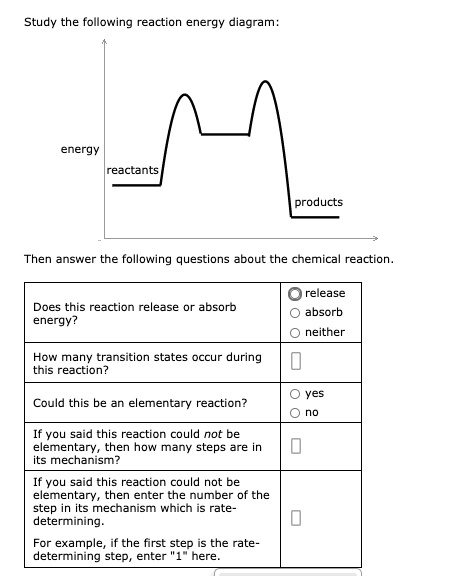
Most reactions in organic chemistry do not proceed in a single step but rather take several steps to yield the desired product. In the course of these multi-step reaction sequences, short-lived intermediates can be generated that quickly convert into other intermediates, reactants, products ...
Which of the following equations correctly defines enthalpy change in terms of internal energy change (U) and gas expansion work (at constant pressure)? ΔH = ΔU + PΔV The reaction below has an enthalpy change equal to Q. Match the modified reactions below with their corresponding enthalpies in terms of Q.
Ask any question and get an answer from our subject experts in as little as 2 hours.
This reaction is represented by the following equation. Ti (s) + O 2 (g) → TiO 2 (s) + 944.0 kJ 20. The formation of titanium (IV) oxide is i , and the reactants have ii potential energy than the products. (K1E, S1A) The statement above is completed by the information in row Row i ii A. endothermic higher B. endothermic lower C. exothermic ...
5. Question 5 - The Energy Diagram of SN2 reaction: Draw an energy diagram for the following S N 2 reaction. Label the axes, the Ea, the ΔH° and the transition state of the reaction.Assume the reaction is exothermic and ΔH° = -75 kJ/mol and Ea = 50 kJ/mol. Draw the structure of reactants and products on the diagram.You can put the reactants at any energy level and then draw the rest as ...
Match each reaction with its correct energy diagram. он он H,SO, H2SO, HCI OH B) C) DIAGRAM 1 DIAGRAM 2 DIAGRAM 3
Potential energy barriers for catalyzed and uncatalyzed reactions. ii. The potential energy diagram compares the potential energy barriers for the catalysed and uncatalysed reactions. The barrier for uncatalysed reaction (E a) is larger than that for the same reaction in the presence of a catalyst E a. iii.
Ask any question and get an answer from our subject experts in as little as 2 hours.
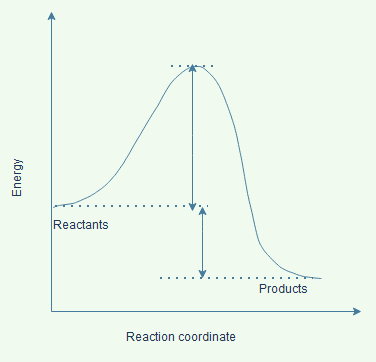
ΔE, net energy change for the reaction. This is the difference in energy between the products and reactants. (Under appropriate conditions, this could also be ΔH.)For this reaction, the energy of products is lower than the energy of reactants, and the reaction releases energy to the surroundings.4. Total potential energy of the products
Study the following reaction energy diagram: products energy reactants Then answer the following questions about the chemical reaction. release Does this reaction release or absorb energy? o absorb o neither How many transition states occur during this reaction? Could this be an elementary reaction?
false. When a chemical reaction takes place, the number of atoms of each element in the reactants ______________ the number of atoms of each element in the products. must be equal to. When a solid compound is formed from chemicals that are in a solution, it is called a precipitate. true.
An enthalpy diagram is a method used to keep track of the way energy moves during a reaction over a period of time. Learn how to draw and label enthalpy diagrams, the definition of an enthalpy ...
Question 47 (1 point) Consider the following reaction energy diagram and choose the statement that is true? Potential Energy Reaction Coordinate O AG is positive and Keg is > 1. O AG is positive and Keg is < 1. O AG is negative and Keg is < 1. O AG is negative and Keg is > 1. Question 48 (1 point) Consider the reaction.














:max_bytes(150000):strip_icc()/catalystenergydiagram-56a12b265f9b58b7d0bcb2fe.jpg)
0 Response to "40 study the following reaction energy diagram:"
Post a Comment