41 t butanol phase diagram
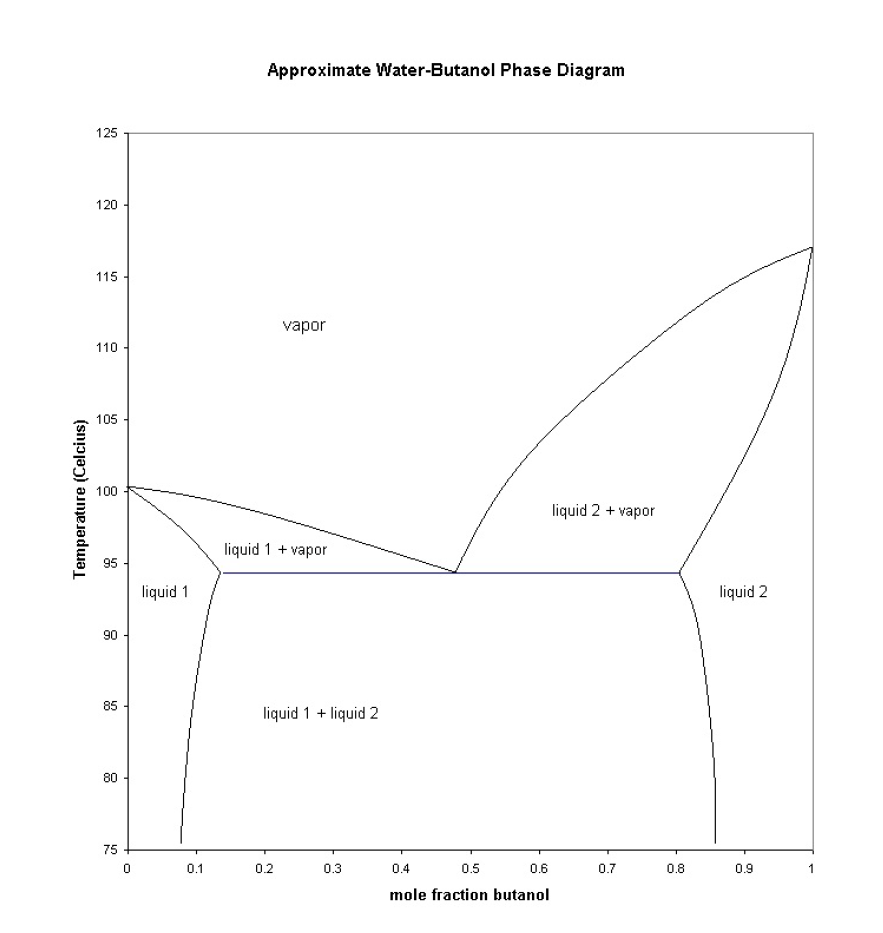
Phase Diagram of tert-Butyl Alcohol. Bill Ponder. Department of Chemistry. Collin College. Phase diagrams are plots illustrating the relationship of ... P-xy T-xy Diagrams. Definition: The P-xy and the T-xy are diagrams that represent the liquid and vapour equilibrium for a binary mixture. The component that is graphed is the most volatile one because is the one that will evaporate first during the distillation process. On the x-axis goes the mole fraction x,y (for liquid phase and vapour phase ...
Fig. 1 shows the P-T phase diagram of tert-butanol and the freeze-drying path applied in this work. ... The slope of this line is very steep because of very small ...

T butanol phase diagram
The phase diagram t-butanol/water is studied in the temperature range between 200 and 450 K at pressures up to 200 MPa. No liquid/liquid phase separation is observed in this range. The solid/liquid phase diagram reveals the presence of a stable t-butanol/dihydrate at all pressures. At the t-butanol rich side of the diagram solid mixtures with ... by BS Bhatnagar · 2020 · Cited by 6 — In order to develop an efficient freeze-drying cycle, the phase behavior under subambient conditions (i.e. in the frozen state) should be understood. In this ... Phase Diagram. and Triple Point of tert-Butanol Experiment. By Michael Heynen & Biruk Gote Boiling Point The. boiling point is the temperature at which the vapor pressure of a liquid equals the pressure of the system As the pressure of the system is reduced, the boiling point of a liquid is lowered. Liquid Gas
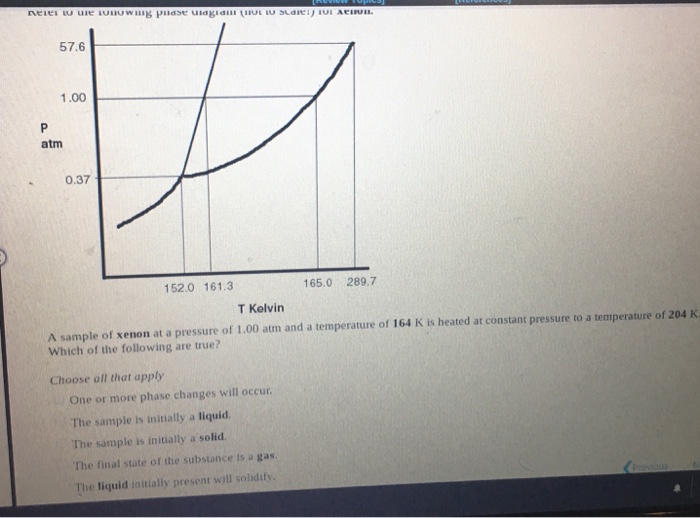
T butanol phase diagram. by M Woznyj · 1985 · Cited by 18 — The phase diagram t-butanol/water is studied in the temperature range between 200 and 450 K at pressures up to 200 MPa. No liquid/liquid phase separation is ... Phase diagram of t-butanol, including labeled triple point, freezing point, and boiling point. This problem has been solved! See the answer See the answer See the answer done loading. Phase diagram of H 2 O with explanations. Phase diagram of CO 2 with explanations. The phase diagram for the said system is developed, described and compared with similar systems studied such as Na 2 S 2 O 3 +ethanol+water, K 2 CO 3 +methanol+water, etc. These results have been explained in terms of structural properties of aqueous t- butanol solutions and further discussed in terms of the effect of ions to cause phase ... by M Woznyj · 1985 · Cited by 18 — The phase diagram t-butanol/water is studied in the temperature range between 200 and. 450 K at pressures up to 200 MPa. No liquid/liquid phase separation ...
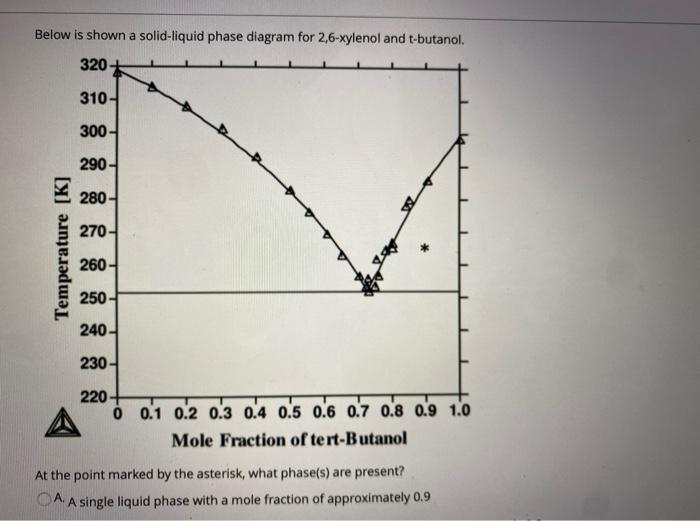
The phase diagram t-butanol/water is studied in the temperature range between 200 and 450 K at pressures up to 200 MPa. No liquid/liquid phase separation is observed in this range. The solid/liquid phase diagram reveals the presence of a stable t-butanol/dihydrate at all pres-sures. A phase diagram (or equilibrium diagram) is a diagram with T and composition as axes, showing the equilibrium constitution. The phase diagram of an alloy made of components A and B, for all combinations of T and X B, defines the A-B system. Binary systems have two components, ternary systems three, and so on. Commercial alloys may by BS Bhatnagar · 2020 · Cited by 6 — While water is the solvent of choice for the lyophilization of pharmaceuticals, tert-butyl alcohol (TBA) along with water can confer several ... Δ H ° fusion = 6.71 kJ/mol. Δ H ° vaporization = 39.1 kJ/mol. csolid = 2.30 J/g °C. cliquid = 3.35 J/g °C. cgas = 1.93 J/g °C. The structure of t-butanol is. In this experiment, you will take temperature readings every 30 seconds (s) from the boiling point to below the freezing point, construct a cooling diagram of temperature vs time ...
The first was a rapid phase, which probably represented the distribution of (14)C-t-butyl alcohol from the blood to other body tissues. The second represented a first-order elimination of radioactivity from the blood with a half-life of approximately 8 hr, indicating that (14)C-t-butyl alcohol was being eliminated primarily as metabolic product(s). Quantity Value Units Method Reference Comment; T boil: 390.6 ± 0.8: K: AVG: N/A: Average of 137 out of 146 values; Individual data points Quantity Value Units Method Reference Comment; T fus: 188. ± 9. Phase Diagram. and Triple Point of tert-Butanol Experiment. By Michael Heynen & Biruk Gote Boiling Point The. boiling point is the temperature at which the vapor pressure of a liquid equals the pressure of the system As the pressure of the system is reduced, the boiling point of a liquid is lowered. Liquid Gas by BS Bhatnagar · 2020 · Cited by 6 — In order to develop an efficient freeze-drying cycle, the phase behavior under subambient conditions (i.e. in the frozen state) should be understood. In this ...

Methyl Ethyl Ketone Water Secondary Butyl Alcohol A Potential System For The Exploration Of A Quadruple Critical Point The Journal Of Chemical Physics Vol 124 No 12
The phase diagram t-butanol/water is studied in the temperature range between 200 and 450 K at pressures up to 200 MPa. No liquid/liquid phase separation is observed in this range. The solid/liquid phase diagram reveals the presence of a stable t-butanol/dihydrate at all pressures. At the t-butanol rich side of the diagram solid mixtures with ...

Equilibrium Data And Thermodynamic Studies Of L Tryptophan Partition In Alcohol Phosphate Potassium Salt Based Aqueous Two Phase Systems Sciencedirect

Thermodynamic Properties And Structure Transition In Water Tert Butanol And Water Tert Butanol Iso Butanol Solutions Sciencedirect

E8 Proppuresub Work Docx Properties Of Pure Substances Name Xinyu Shen Date Results Observations Part A Phase Behavior Of Water 1 What Happened When A Course Hero

Mechanism And Temperature Dependent Kinetics Of The Dehydration Of Tert Butyl Alcohol In Hot Compressed Liquid Water Industrial Engineering Chemistry Research

Differential Scanning Calorimetry Studies Of Phospholipid Membranes The Interdigitated Gel Phase Intechopen
Pengukuran Kesetimbangan Uap Cair Sistem Biner Isothermal 2 Butanol Dietil Karbonat Dan Tert Butanol Dietil Karbonat Pada Temperatur 303 15 323 15 K Its Repository

Tert Butanol Water Mixture Separation By Extractive Distillation Application Of Experimental Data In Process Simulations Sciencedirect
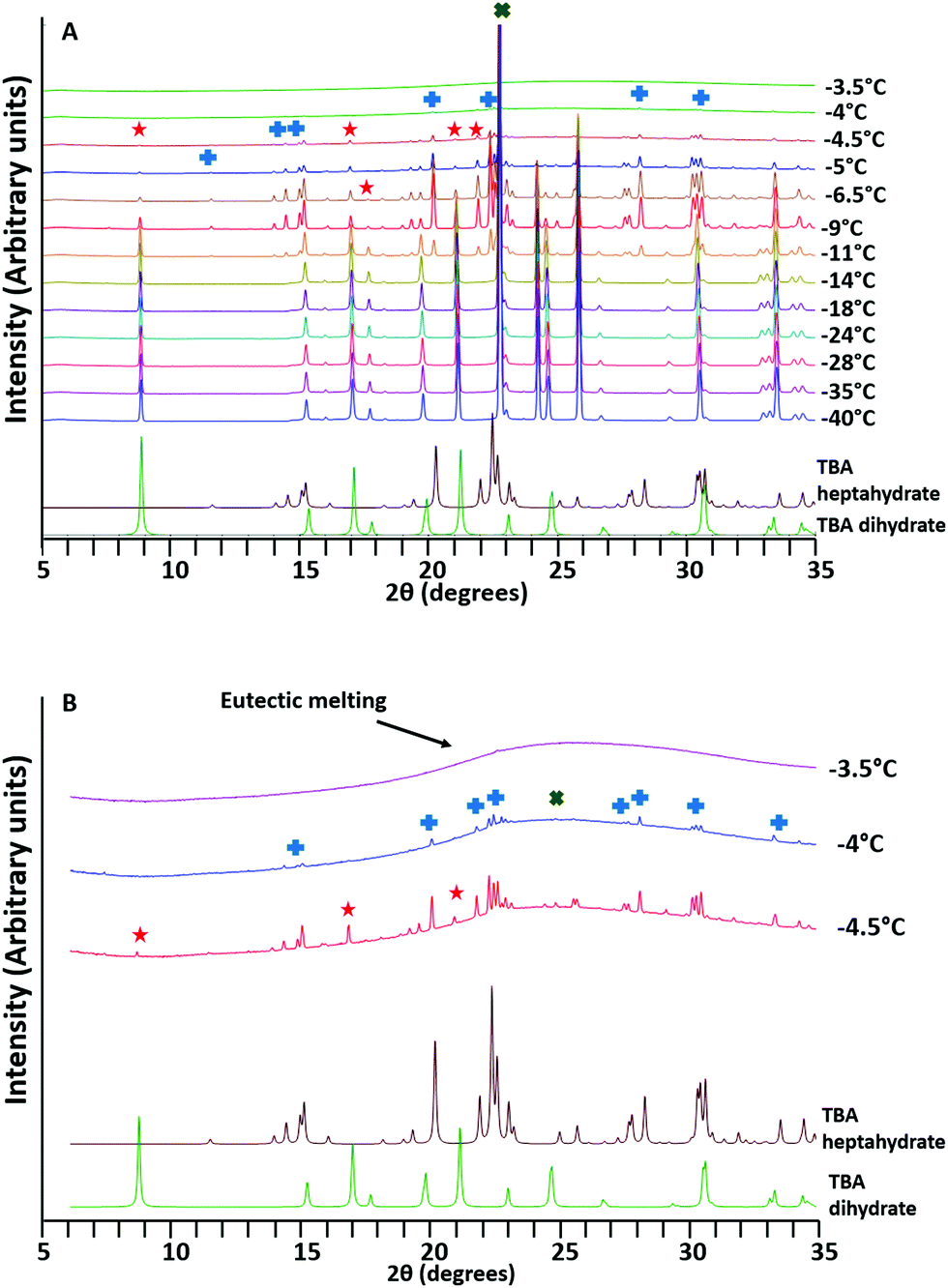
A Refined Phase Diagram Of The Tert Butanol Water System And Implications On Lyophilization Process Optimization Of Pharmaceuticals Physical Chemistry Chemical Physics Rsc Publishing

Isobaric Vapour Liquid Equilibrium Of Tert Butanol Water System With Biological Buffer Tris At 101 3 Kpa Sciencedirect
Use Of Aqueous Two Phase And Three Phase Partitioning Systems For Purification Of Lipase Obtained In Solid State Fermentation By Rhizopus Arrhizus
Molecules Free Full Text Phenolic Composition Antioxidant And Anticancer Potentials Of Extracts From Rosa Banksiae Ait Flowers Html

Pdf Analysis Of Tert Butanol Methyl Tert Butyl Ether Benzene Toluene Ethylbenzene And Xylene In Ground Water By Headspace Gas Chromatography Mass Spectrometry Semantic Scholar











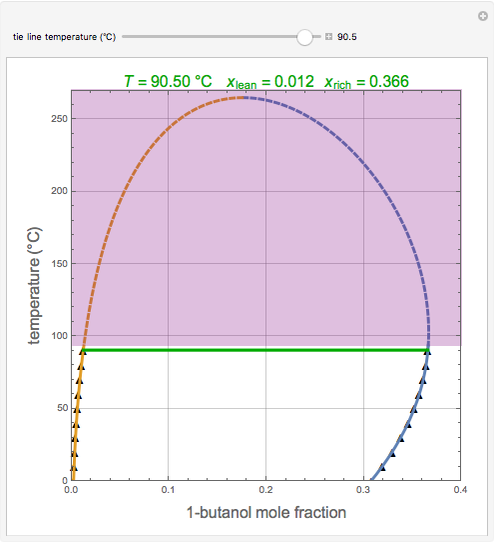


0 Response to "41 t butanol phase diagram"
Post a Comment