39 what is an energy diagram
Energy Photosynthesis works as a primary source of energy that is vital to keep things alive. How to Create a Photosynthesis Diagram Creating a photosynthesis diagram is fairly simple and straightforward as long as you understand how the process works. An energy flow diagram (or Sankey) tracks the energy flow from raw or primary sources of energy up to the final stage (consumption). In other words, a Sankey Diagram is a directional flow chart where the width of the streams is proportional to the quantity of flow.
What is energy level diagram? In chemistry, an electron shell, or energy level, may be imagined as an orbit with electrons around the nucleus of an atom. The closest shell to the nucleus is called the "K shell" followed by the "L shell" then the "M shell" and so on away from the nucleus. The shells can be denoted by alphabets (K, L ...
What is an energy diagram
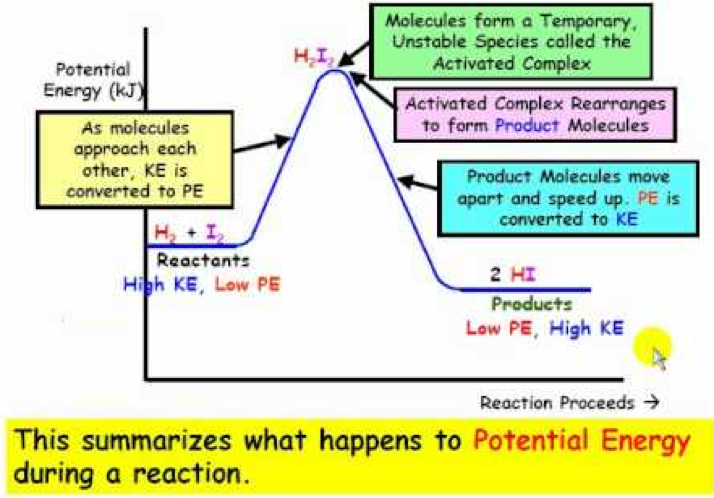
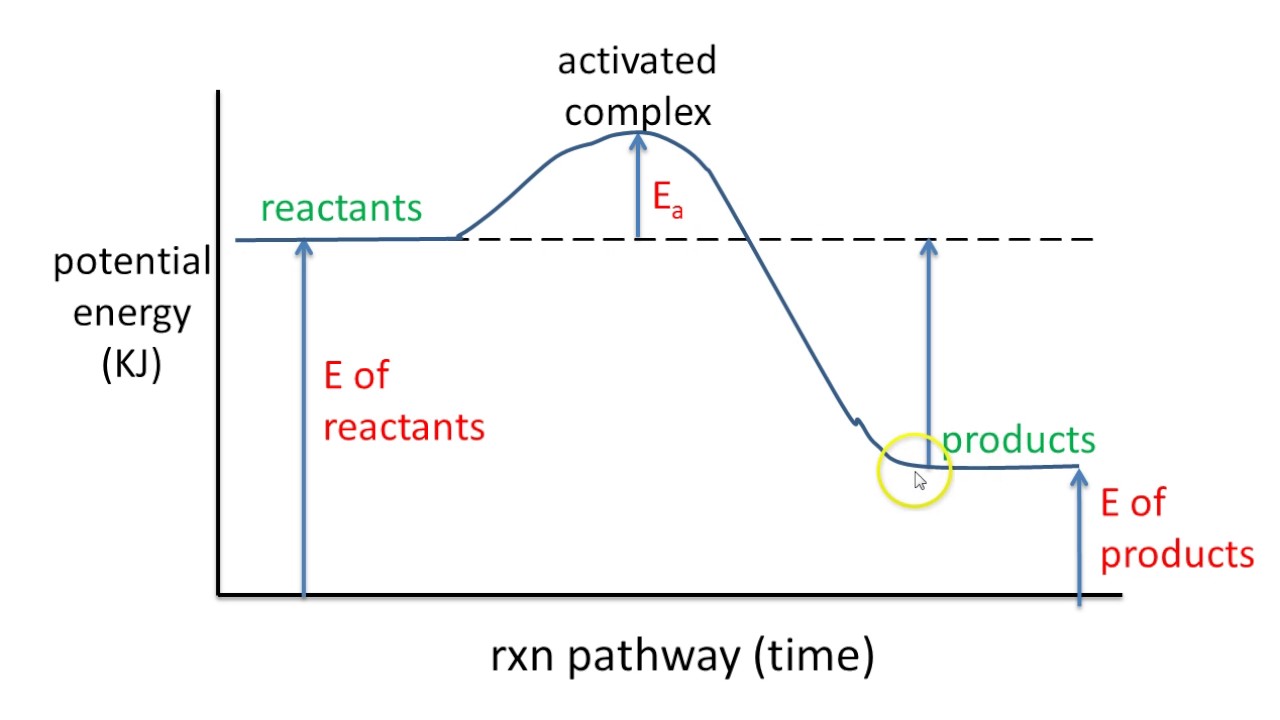
A Energy diagram Is a graph of energy that illustrates the process that occurs throughout a reaction. The energetic diagrams can also be defined as the visualization of an electronic configuration in orbitals; Each representation is an electron of an orbital with an arrow. Energy level diagrams are used to shows the energy content of chemicals before and after a reaction. They show: (a) the total energy content of the reactants compared to the total energy content of the products. Figure shows the energy level diagram of an exothermic reaction. Figure shows the energy level diagram of an endothermic reaction. 21/11/2010 · The energy difference between any two adjacent levels gets smaller as n increases, which results in the higher energy levels getting very close and crowded together just below n = ∞ . The ionization energy of an atom is the energy required to remove the electron completely from the atom.(transition from ground state n = 0 to infinity n = ∞ ).
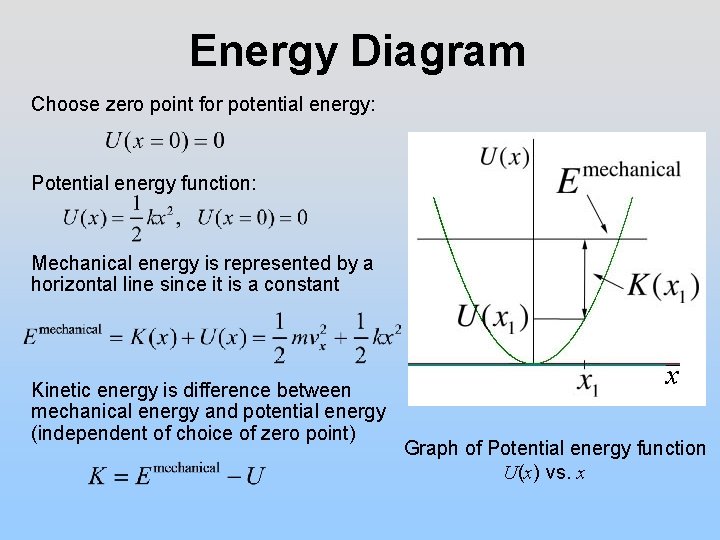
What is an energy diagram. An energy cycle is a diagram showing how three, or more reactions are interconvertable. Energy cycles can be constructed to find unknown energy changes, by adding up the energies of steps from a different route to the same final products. The overall change in energy in a reaction is the difference between the energy of the reactants and products. Exothermic reactions The diagram shows a reaction profile for an exothermic reaction. Potential energy diagrams Consider an arbitrary potential energy shown schematically below. There are a number of important qualitative features of the behavior of the system that can be determined by just knowing this curve. The first thing to notice is that since the kinetic energy Interpreting a one-dimensional potential energy diagram allows you to obtain qualitative, and some quantitative, information about the motion of a particle. At a turning point, the potential energy equals the mechanical energy and the kinetic energy is zero, indicating that the direction of the velocity reverses there.
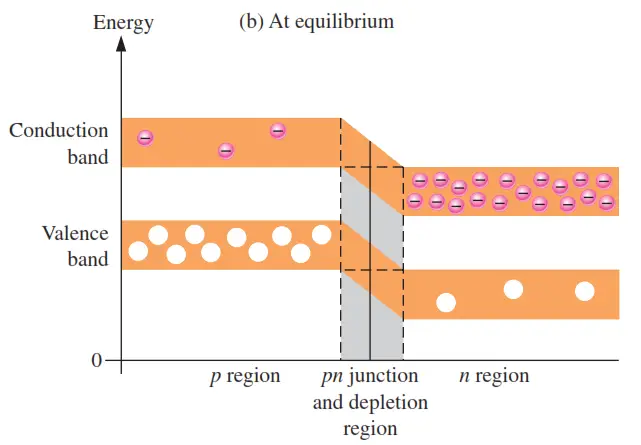
Band structures are a representation of the allowed electronic energy levels of solid materials and are used to better inform their electrical properties. A band structure is a 2D representation of the energies of the crystal orbitals in a crystalline material. Sometimes referred to as "spaghetti diagrams," a band structure plot can Answer (1 of 2): Just as a flat map represents the relationship of continents on a sphere an energy diagram details the relationship between stable electron energy levels around an atom as flat lines. So before you can understand the map you've got to understand the thing being mapped. We are m... The diagram shows the energy band diagram of pure semiconductor at room temperature, the electrons are present in the valence band as shown. Now at high temperature or by receiving some additional energy the electron from valence band jumps to the conduction band. Phase diagrams. Enthalpy. Heat of formation. Hess's law and reaction enthalpy change. Gibbs free energy and spontaneity. Gibbs free energy example. More rigorous Gibbs free energy / spontaneity relationship. A look at a seductive but wrong Gibbs spontaneity proof. Endothermic vs. exothermic reactions. This is the currently selected item.
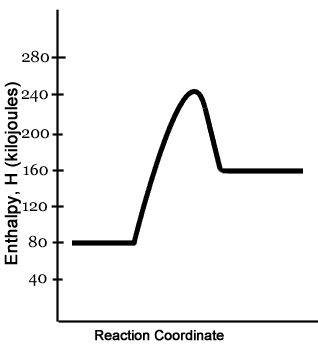
energy diagram . These diagrams can be useful in describing motion for all types of objects. Once you have the potential energy diagram for a situation, you can describe the motion of an object. The usual method of using potential energy diagrams is to: • start with the physical situation • use the physics to draw the potential energy diagram This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f... An enthalpy diagram plots information about a chemical reaction such as the starting energy level, how much energy needs to be added to activate the reaction, and the ending energy. An enthalpy ... Energy diagrams are diagrams that show the amount of relative potential energy in each step of a reaction · Energy diagrams typically include: the reactants, ...
11/04/2019 · Energy, Water, and Carbon Informatics. Lawrence Livermore National Laboratory (LLNL) produced the first diagrams illustrating U.S. national commodity use in the mid-1970s. The most widely recognized of these charts is the U.S. energy flow chart.
Energy Pyramid is a popular diagramming model that describes the flow of energy (its production and consumption) at different ecological levels.An energy pyramid diagram would depict the flow of biomass or bio-productivity at various trophic stages. Now, with the help of a diagramming tool like EdrawMax, anyone can create visually appealing energy pyramid diagrams on the go.
The energy band diagram of semiconductor is shown where the conduction band is empty and the valence band is completely filled but the forbidden gap between the two bands is very small that is about 1eV. For Germanium, the forbidden gap is 0.72eV and for Silicon, it is 1.1eV. Thus, semiconductor requires small conductivity.
Jul 9, 2019 — Potential Energy Diagrams. The energy changes that occur during a chemical reaction can be shown in a diagram called a potential energy ...
Jul 9, 2021 — The energy changes that occur during a chemical reaction can be shown in a diagram called a potential energy diagram, or sometimes called a ...
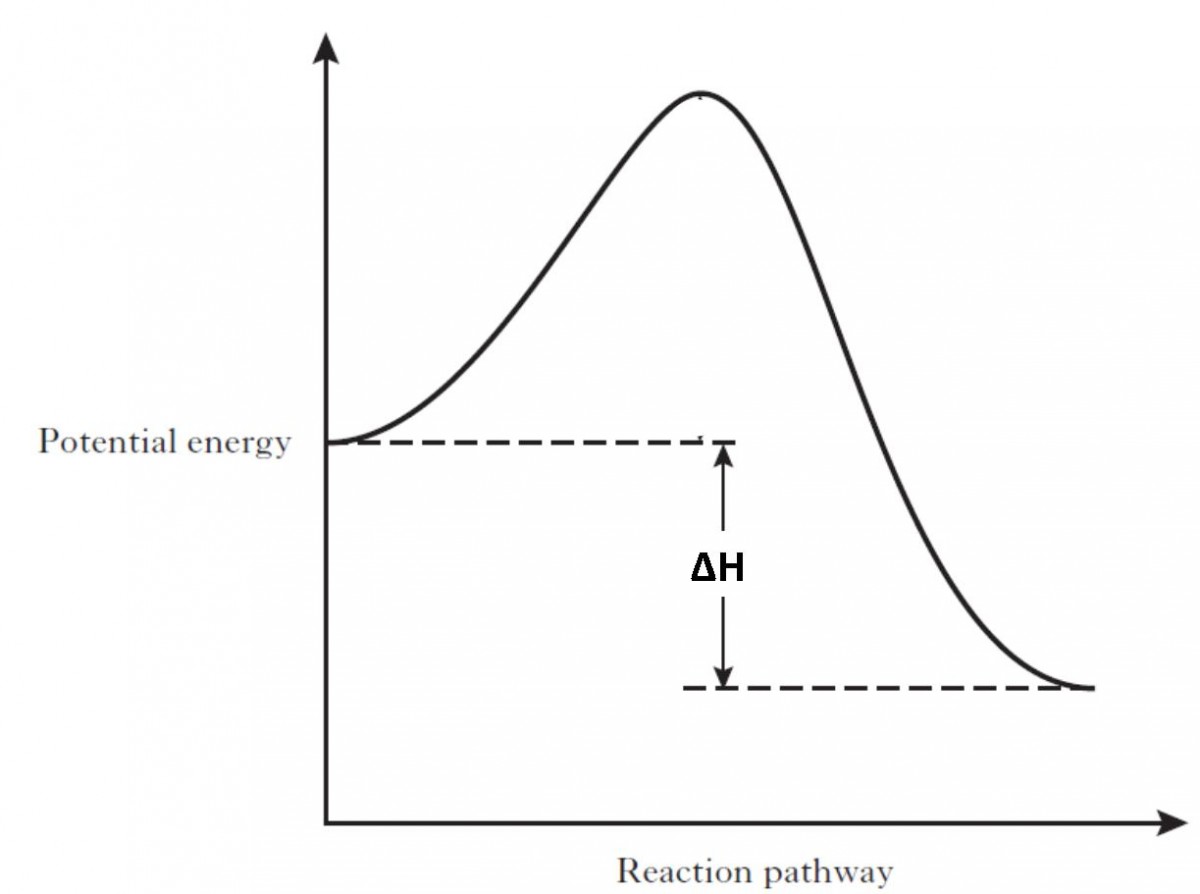
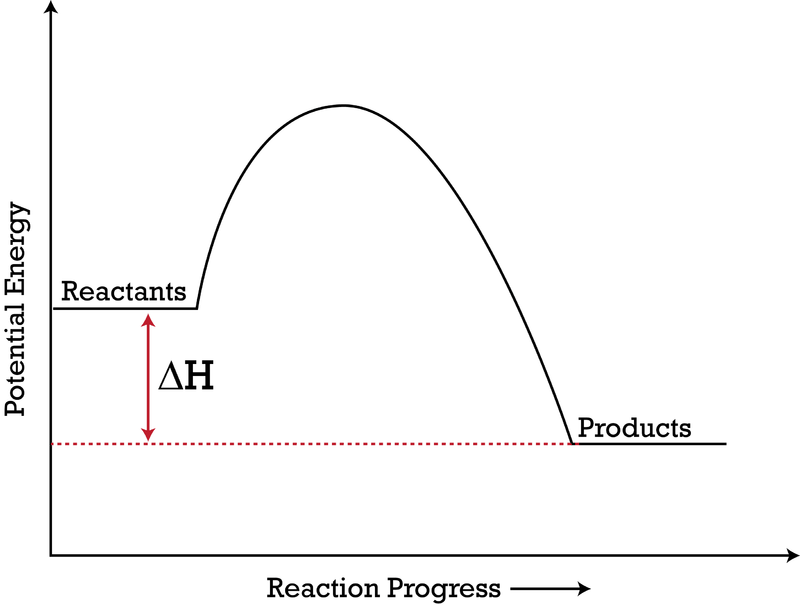
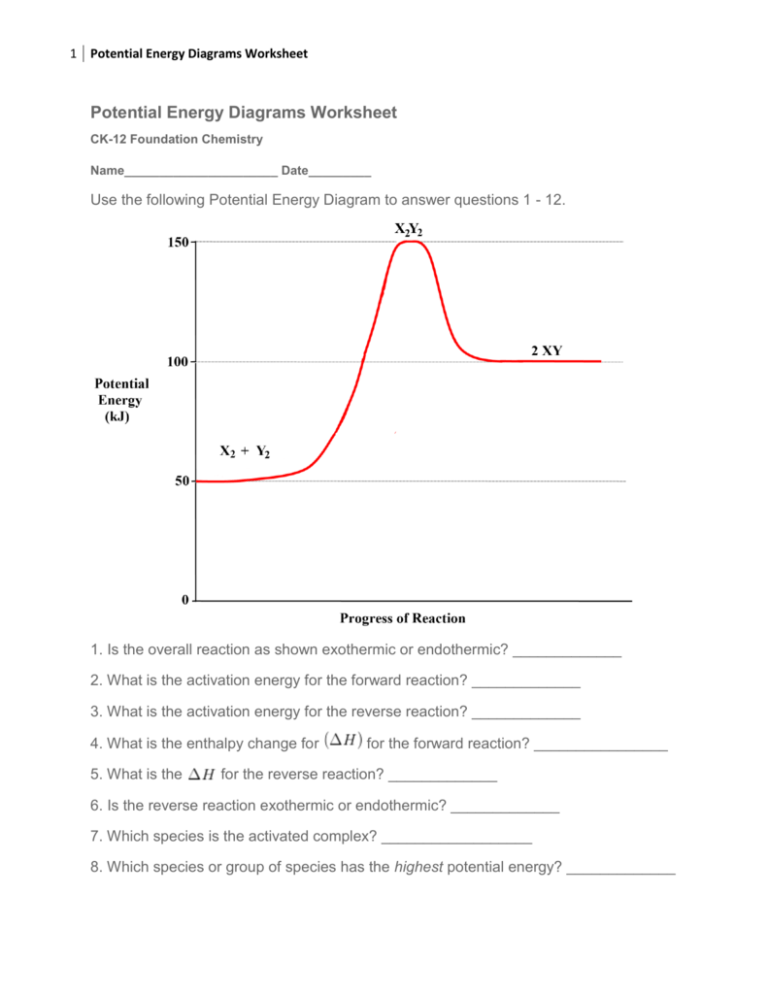
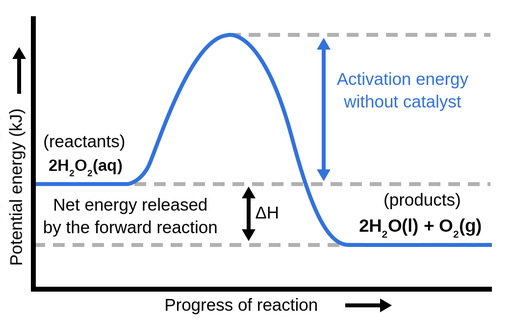
Δ H is the overall enthalpy change for a reaction. Potential energy diagrams can be used to calculate both the enthalpy change and the activation energy for a ...
Reaction coordinate diagrams — For a chemical reaction or process an energy profile (or reaction coordinate diagram) is a theoretical representation of a ...
27/05/2021 · Glycolysis is the only source of energy in erythrocytes. When performing physically-demanding tasks, muscle tissues may experience an insufficient supply of oxygen, the anaerobic glycolysis serves as the primary energy source for the muscles.
Answer (1 of 2): An energy level diagram is exactly what it says: it is a diagram showing levels of energy. In quantum mechanics, it is a graphical representation of ...
A Jablonski diagram is basically an energy diagram, arranged with energy on a vertical axis. The energy levels can be quantitatively denoted, but most of these diagrams use energy levels schematically. The rest of the diagram is arranged into columns. Every column usually represents a specific spin multiplicity for a particular species.
The earth-atmosphere energy balance is the balance between incoming energy from the Sun and outgoing energy from the Earth. Energy released from the Sun is emitted as shortwave light and ultraviolet energy. When it reaches the Earth, some is reflected back to space by clouds, some is absorbed by the ...
6.7: Energy Diagrams. You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams. In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ' reaction coordinate ', tracing from left to right the progress of the reaction ...
In solid-state physics of semiconductors, a band diagram is a diagram plotting various key electron energy levels (Fermi level and nearby energy band edges) as a function of some spatial dimension, which is often denoted x. These diagrams help to explain the operation of many kinds of semiconductor devices and to visualize how bands change with position (band bending).
Phase Change Diagram - Flat Line • Anytime there is a flat line on a temp-energy graph, a phase change is occurring.
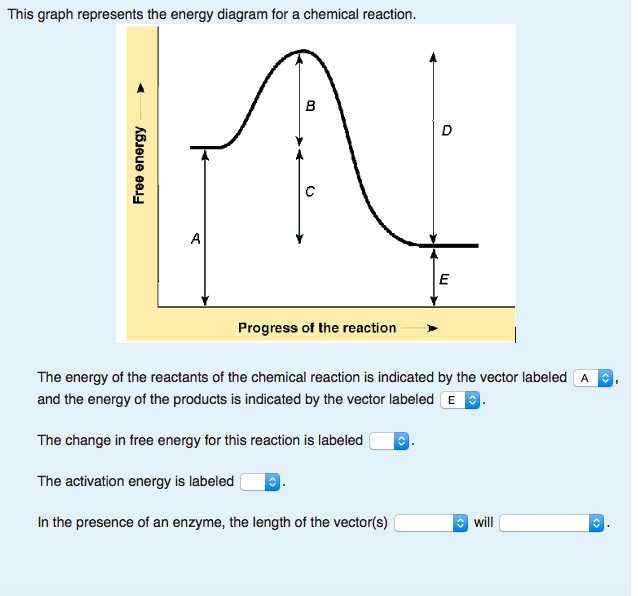
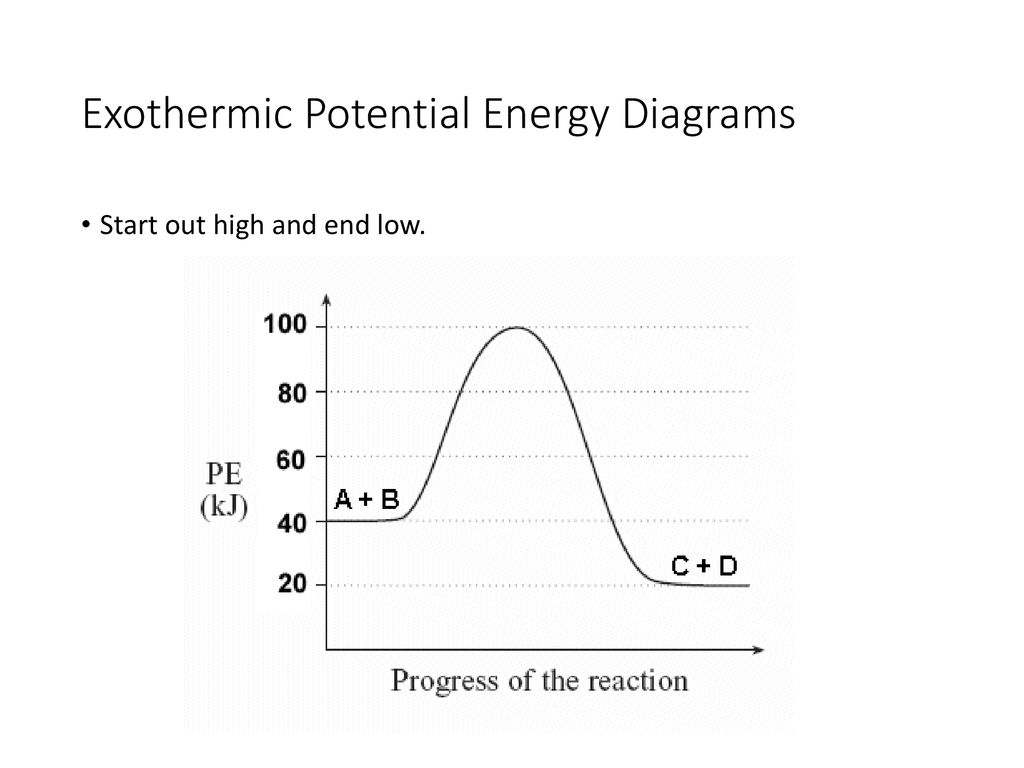
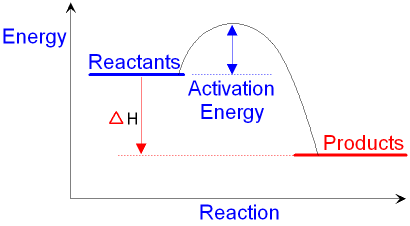
The reaction shown by the second diagram is more exothermic. There is a greater difference in energy between the reactants and products. The green arrow is longer.
Solar energy production encompasses several power sources, both passive and active. It's important to differentiate among the different types of solar energy production systems since it's not uncommon for the average homeowner to confuse them. We'll start with a diagram of solar energy hitting the earth surface. Then we'll present diagrams and discuss photovoltaic solar,…
Sankey diagrams are a type of flow diagram in which the width of the arrows is proportional to the flow rate.. The illustration shows a Sankey diagram that represents all the primary energy that flows into a factory. The widths of the bands are linearly proportional to energy production, utilization and loss.
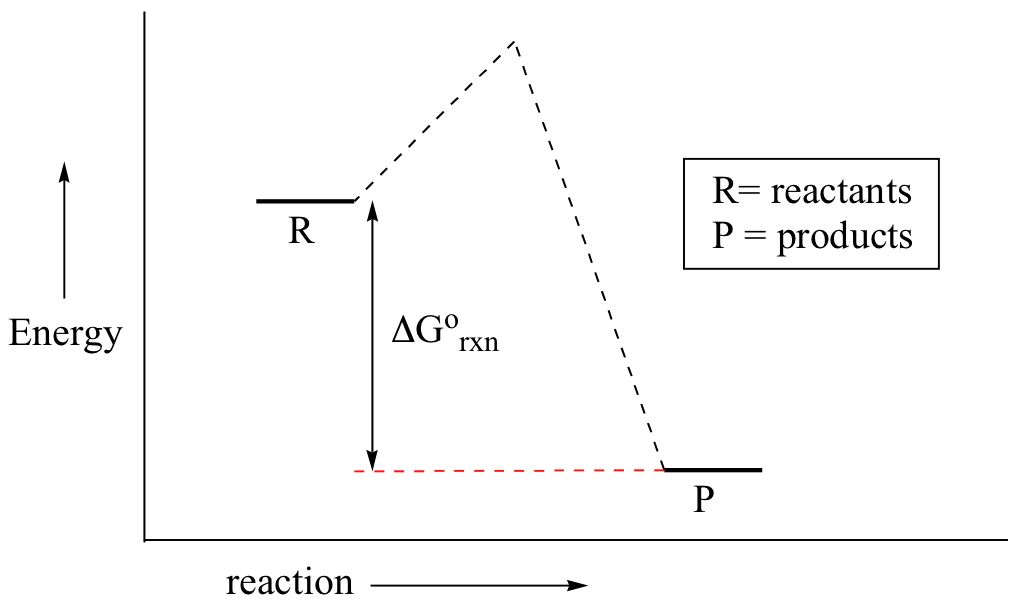
Reaction coordinate diagrams for exergonic and endergonic reactions. In the exergonic reaction, the reactants are at a higher free energy level than the products (reaction goes energetically downhill). In the endergonic reaction reaction, the reactants are at a lower free energy level than the products (reaction goes energetically uphill).
The PV diagram models the relationship between pressure (P) and volume (V) for an ideal gas.An ideal gas is one that never condenses regardless of the various changes its state variables (pressure, volume, temperature) undergo. In addition, the processes plotted on PV diagrams only work for a closed system (in this case the ideal gas), so there is no exchange of matter, but still an exchange ...
A potential energy diagram plots the change in potential energy that occurs during a chemical reaction. This first video takes you through all the basic parts of the PE diagram. Sometimes a teacher finds it necessary to ask questions about PE diagrams that involve actual Potential Energy values.
An energy profile is a diagram representing the energy changes that take place during a chemical reaction. Enthalpy change , ΔH, is the amount of energy absorbed or released by a chemical reaction. On an energy profile, the enthalpy change for the reaction is measured from the energy of the reactants to the energy of the products.
Energy Diagrams. Exothermic Reactions. Endothermic Reactions. Example. 6.3 Kinetic Energy, Heat Transfer, and Thermal Equilibrium. 6.4 Heat Capacity and Coffee-Cup Calorimetry. 6.5 Phase Changes and Energy. 6.6 Introduction to Enthalpy of Reaction. 6.7 Bond Enthalpy and Bond Dissociation Energy.
An energy flow diagram is a visual way to show the uses of energy in a system. A simple diagram for the energy flow in a mobile phone is shown below. This shows how energy is converted from one form into another. The usable energy stored in the battery as chemical energy becomes degraded energy when
Energy Flow Charts. A single energy flow chart depicting resources and their use represents vast quantities of data. Energy resources included solar, nuclear, hydroelectric, wind, geothermal, natural gas, coal, biomass, and petroleum. Energy flow diagrams change over time as new technologies are developed and as priorities change.
The figure shows the energy band diagram of the silicon atom. Silicon atom has 14 electrons as silicon atomic number is 14. All 14 electrons are distributed in 3 orbits. All energy level is associated with a certain amount of energy. And all bands are separated from the adjacent bands by the energy gap.No electron can exist in the energy gap.
Energy diagrams depict the reaction progress versus energy. For exothermic reactions, the reactants are drawn above the products because their energy is ...Dec 22, 2010
The energy band diagram of the conductor is shown below. energy-band-in-conductors. The main characteristics of conductors mainly include the energy gap like forbidden will not exist. The energy bands like valance as well as conduction will get overlapped. The availability of free electrons for conduction is ample.
21/11/2010 · The energy difference between any two adjacent levels gets smaller as n increases, which results in the higher energy levels getting very close and crowded together just below n = ∞ . The ionization energy of an atom is the energy required to remove the electron completely from the atom.(transition from ground state n = 0 to infinity n = ∞ ).
Energy level diagrams are used to shows the energy content of chemicals before and after a reaction. They show: (a) the total energy content of the reactants compared to the total energy content of the products. Figure shows the energy level diagram of an exothermic reaction. Figure shows the energy level diagram of an endothermic reaction.
A Energy diagram Is a graph of energy that illustrates the process that occurs throughout a reaction. The energetic diagrams can also be defined as the visualization of an electronic configuration in orbitals; Each representation is an electron of an orbital with an arrow.

















0 Response to "39 what is an energy diagram"
Post a Comment