41 orbital diagram for germanium
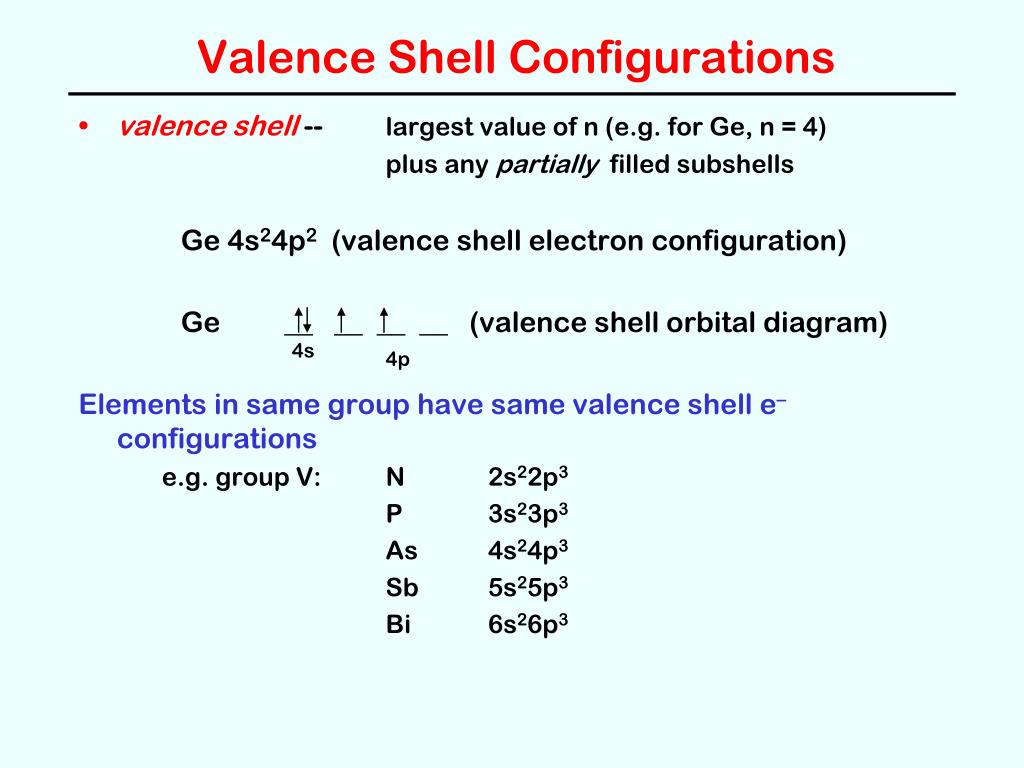
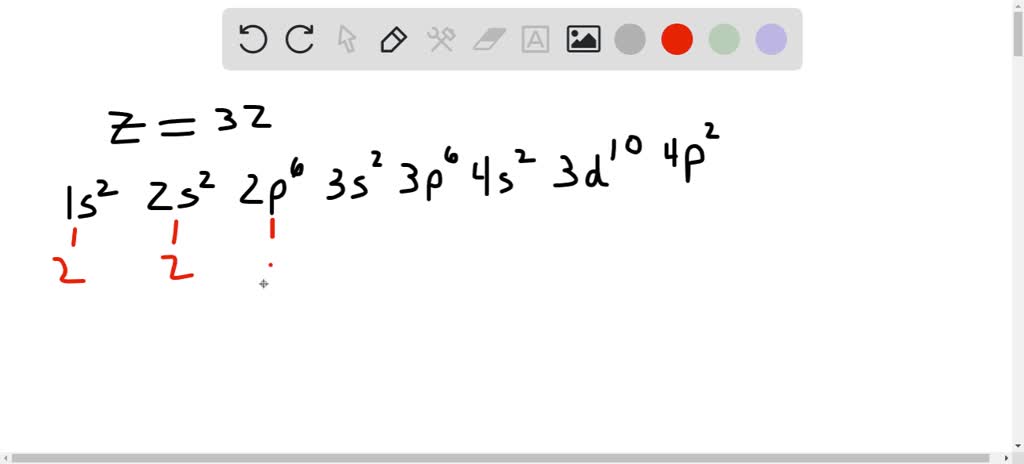
Electronic Configuration of Ge (Germanium) Atomic number of germanium is 32. Its electronic configuration is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p2 or it can be written as [Ar] 3d10 4s2 4p2. ... Orbital Diagram 1s ↿⇂ 2s ↿⇂ 2p ↿⇂ ↿⇂ ↿⇂ 3s ↿⇂ 3p ↿⇂ ↿⇂ ↿⇂ 3d ↿⇂ ↿⇂ ↿⇂ ↿⇂ ↿⇂ 4s ↿⇂ 4p ... Germanium Orbital Diagram. Electron Configurations and Orbital Diagrams KEY. Draw orbital diagrams for the following elements: 1. phosphorus. ↑↓. ↑↓ 4. germanium. ↑↓. ↑↓. Mendeleev's Predicted Properties of Germanium ("eka. Silicon") and Its Use the orbital diagram to find the third and eighth electrons. PROBLEM: Write a set ...
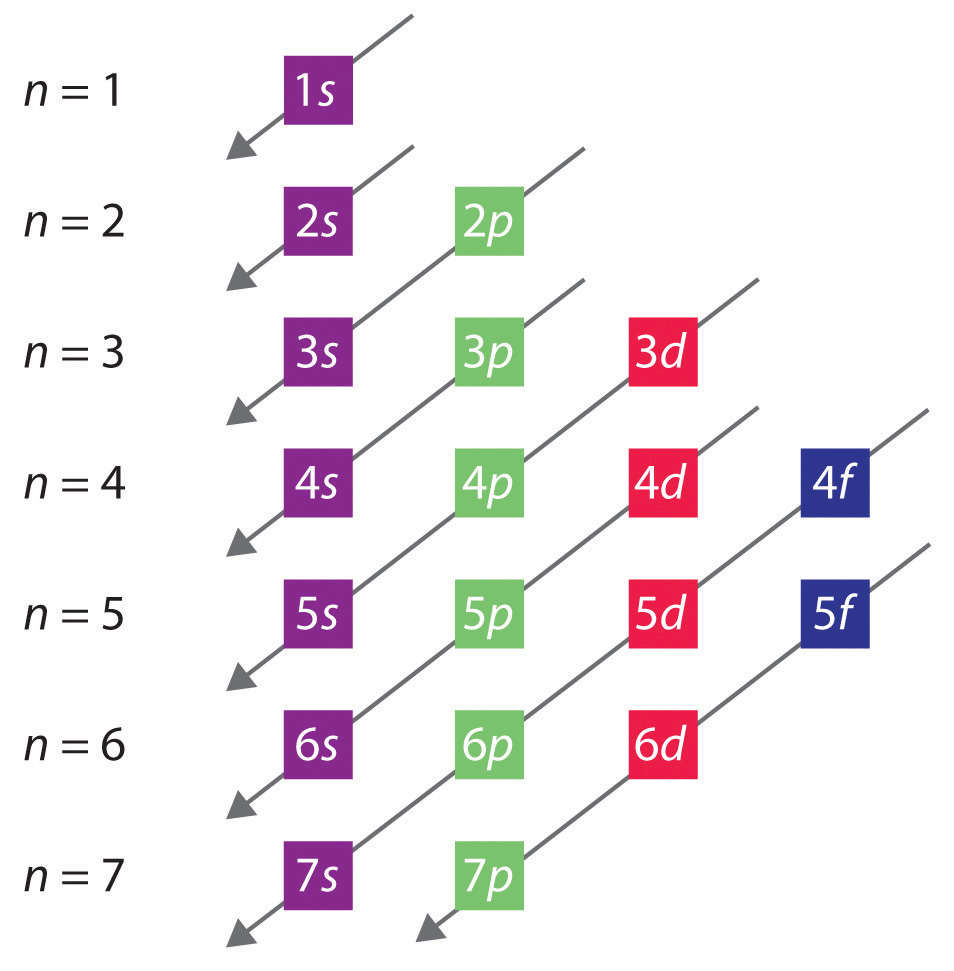
Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau...
Orbital diagram for germanium
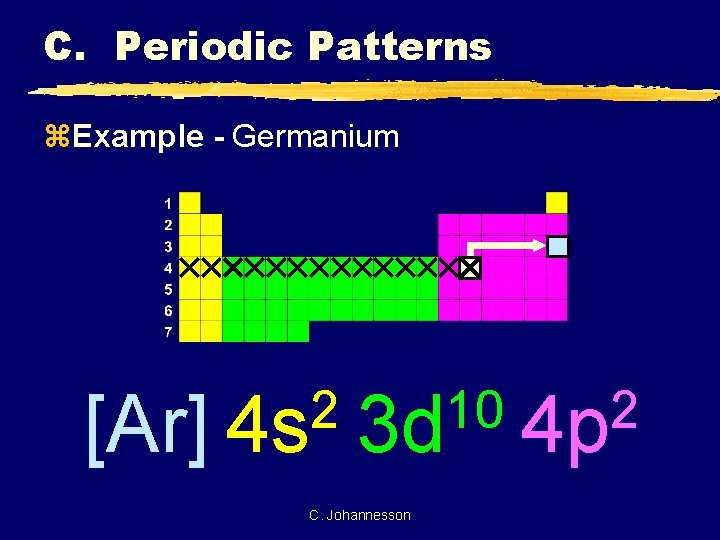
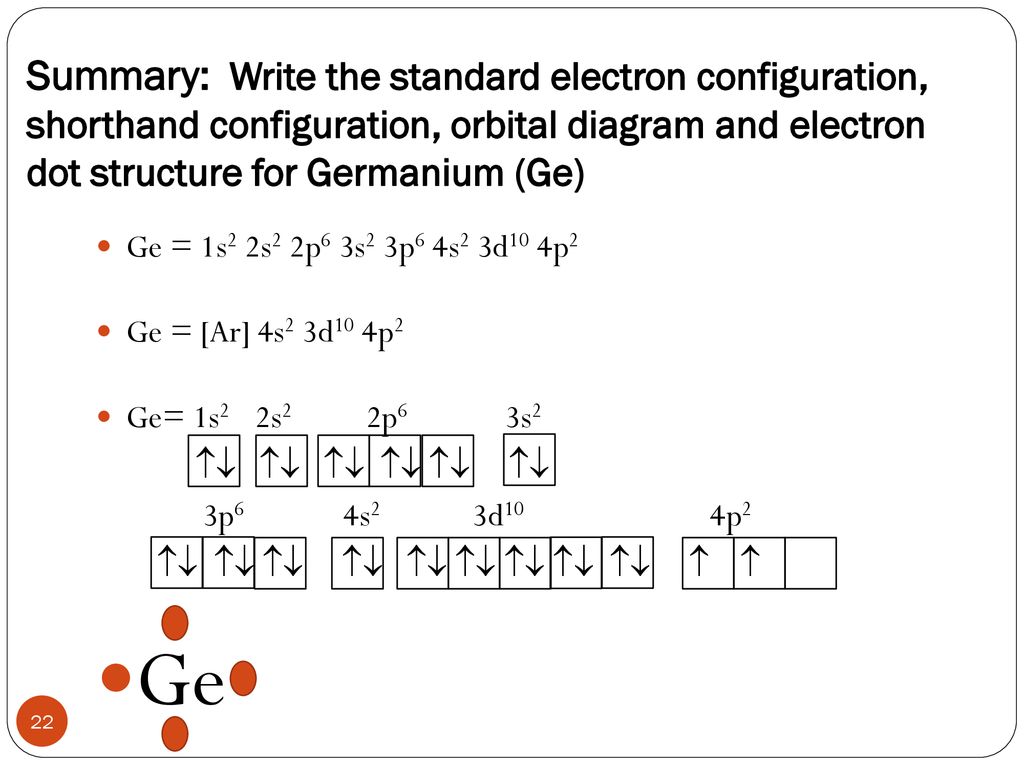
1. The Pauli principle: No more than two electrons can occupy a given orbital. If there are two electrons in an orbital, their spins must be paired (one must have m s = 1 2 and the other, m s = − 1 2). 2. The aufbau (building-up) principle: When electrons are filled in to orbitals in an atom, the orbitals with lower energy are filled first. Germanium (Ge) is located in the fourth row, group 14 of the periodic table, and has an atomic number of 32. This implies that the neutral Ge atom's electron configuration must account for 32 electrons. So, "Ge": 1s^(2)2s^(2)2p^(6)3s^(2)3p^(6)4s^(2)3d^(10)4p^(2) An alternative way of writing the electron configuration for Ge is by using the noble gas shorthand notation. 0 Comments. on Orbital Diagram For Germanium. orbital. Because an electron can have either one of two spins, any orbital can hold a maximum of four . The orbital diagram for germanium is. 1s. 2s. 2p. 3s. Oxidation States, +4,2. Electrons Per Shell, 2 8 18 4. Electron Configuration, [Ar] 3d10 4s2 4p2. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p2.
Orbital diagram for germanium. This page contains materials for the session on the band theory of solids. It features a 1-hour lecture video, and also presents the prerequisites, learning objectives, reading assignment, lecture slides, homework with solutions, and resources for further study. 13. The orbital diagram for a ground-state oxygen atom is. 14. The orbital diagram for a ground state carbon atom is. 15. Which ground-state atom has an electron configuration described by the following orbital diagram? A) phosphorus B) germanium C) selenium D) tellurium E) none of these. 16. In the case of Germanium the abbreviated electron configuration is [Ar] 3d10 4s2 4p2. Nevertheless, check the complete configuration and other interesting facts about Germanium that most people don't know. Germanium Overview Germanium Complete Electron Configuration 1s2 2s2 2p6 3s2 3p6 4 s2 3 d10 4 p1 Abbreviated Electron Configuration [Ar ... Answer (1 of 2): First of all, I hate when this happens! Your teacher is actually wrong. The electron configuration of Ge2+ is [Ar] 4s2 3d10 Below I drew out exactly what will happen when you remove (+2) 2 electrons from the Germanium ion (Ge). If you want to use a noble gas to configure an ion,...
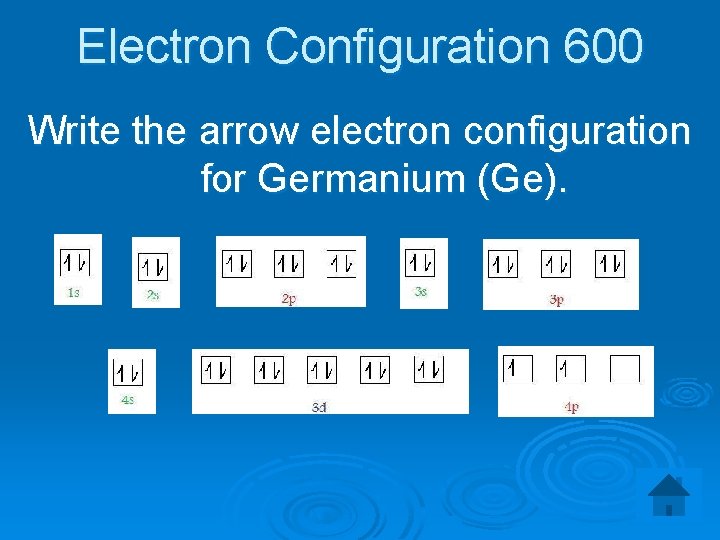
Use the orbital-filling diagram to show the electron configuration of helium, He. ... germanium strontium phosphorus silver molybdenum. phosphorus germanium silver molybdenum strontium. Arrange the following elements in order of decreasing metallic character (high to low): Cl Cs Sr Rh Se Mo As Cd. Cs Sr Mo Rh Cd As Se Cl. Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom The orbital diagram for a ground state carbon atom is. Ans: D. Category: Medium Section: 7.8. 43. Which ground-state atom has an electron configuration described by the following orbital diagram? A) phosphorus B) germanium C) selenium D) tellurium E) none of these. Ans: C Category: Medium Section: 7.9. 44. Which ground-state atom has an ... An orbital is a region of space that an electron can exist in. For the diagram you start with the 1 s orbital and then 2s, 2p, and so on. Each orbital can hold 2 electrons and each arrow ...
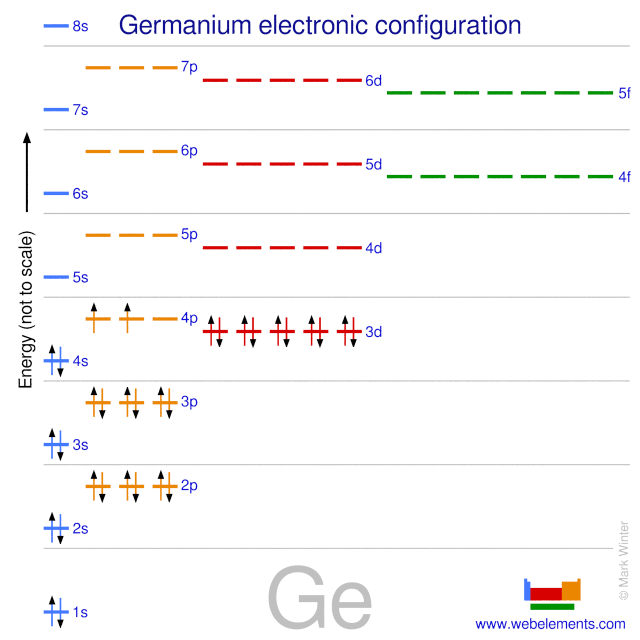
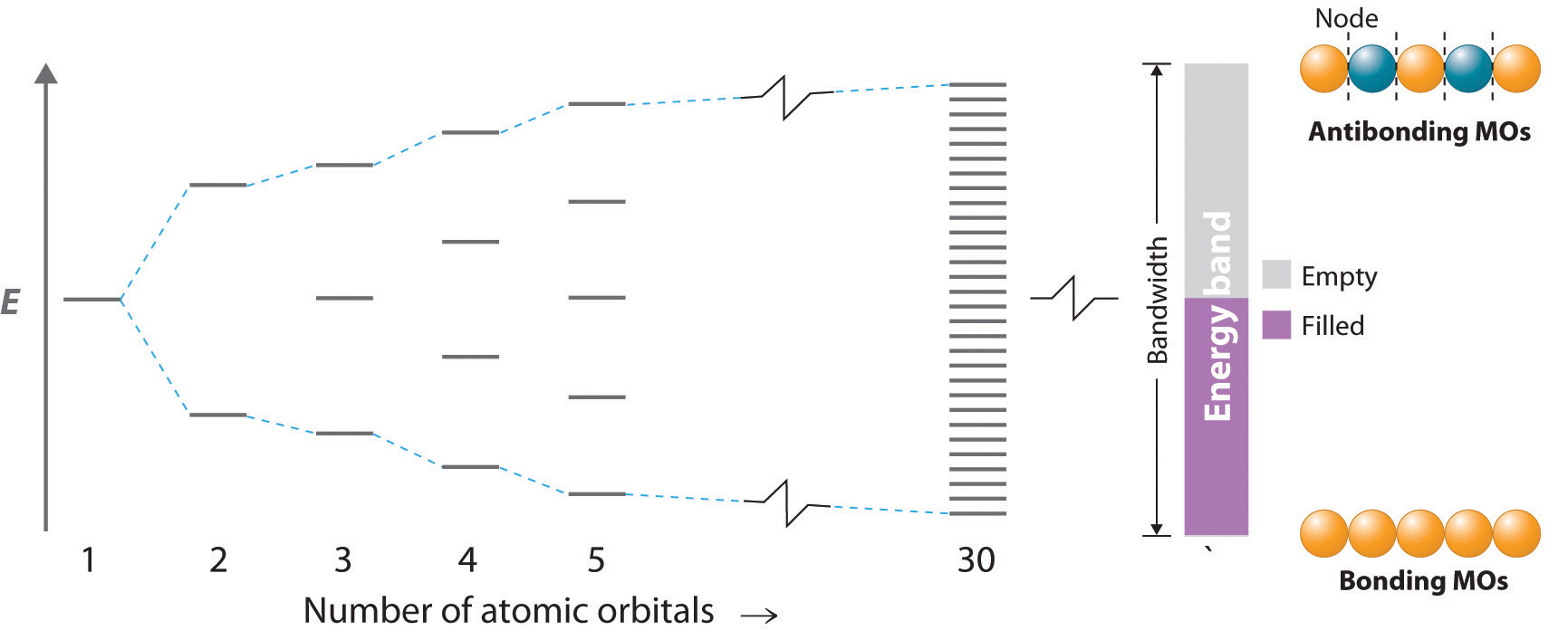
Compound properties. Element reactions. Germanium atoms have 32 electrons and the shell structure is 2.8.18.4. The ground state electron configuration of ground state gaseous neutral germanium is [ Ar ]. 3d10. 4s2. 4p2 and the term symbol is 3P0. Schematic electronic configuration of germanium. The Kossel shell structure of germanium. Figure 12.21 The Molecular Orbital Energy-Level Diagram for a Linear Arrangement of n Atoms, Each of Which Contains a Singly Occupied s Orbital. This is the same diagram as Figure 9.35 "Bonding in Ozone", with the addition of the far right-hand portion, corresponding to n = 30 and n = ∞. As n becomes very large, the energy separation between adjacent levels becomes so small that a single ... Germanium. Full electron configuration of germanium: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p2. gallium ← germanium → arsenic. The orbital diagram for germanium is. The orbital filling diagram for helium The electron configuration for helium is 1s². The symbol of lead is Pb lead has an Atomic Number of 82. If we gave you brief information then the first two electrons lie in the 1s orbital following the next 2 electrons it comes under the 2s orbital.
Germaine is a hazardous substance UN2192 which is classified as a poisonous gas (2.3). It is also a flammable gas (2.1). Other compounds include: Germanium dichloride Ge Cl 2, Germanium dioxide GeO 2, Germanium tetrachloride GeCl 4 this is very irritating to eyes and membranes. Germanium Menu. Germanium Page One. Overview of Germanium
The electron configuration notgtion and orbital diagram for sodium are written Na Is22s22pK3s 1s2s Noble-gas notation is a method of representing electron configurations of ... Germanium (Ge). a semiconducting element, is commonly used in the manufacture of computer chips. What is the ground-state electron
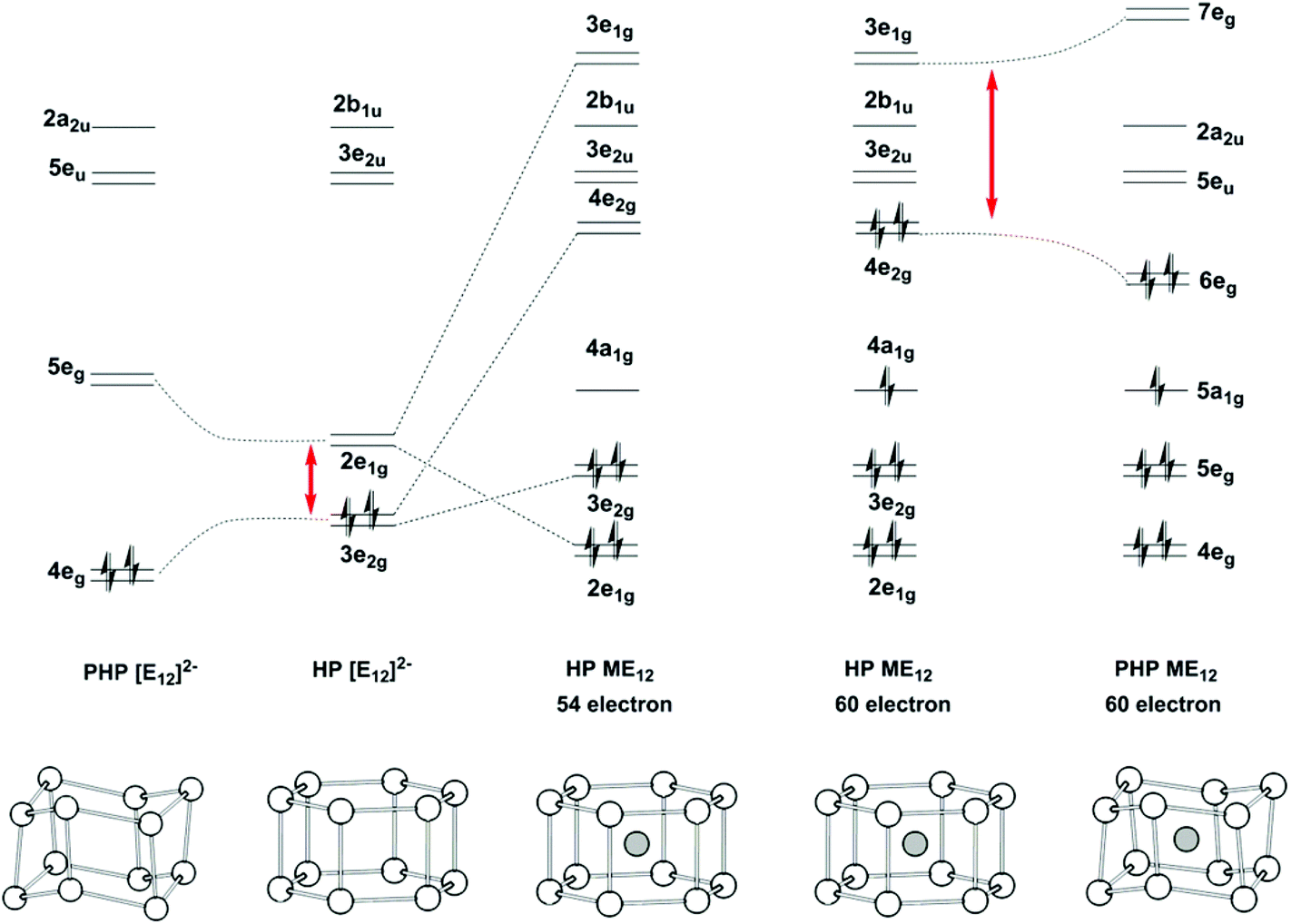
On The Structural Landscape In Endohedral Silicon And Germanium Clusters M Si 12 And M Ge 12 Dalton Transactions Rsc Publishing Doi 10 1039 C4dt03573a
The orbital diagram for a ground-state nitrogen atom is A) A B) B C) C D) D see chart. A. The orbital diagram for a ground-state oxygen atom is ... Which ground-state atom has an electron configuration described by the following orbital diagram? A) phosphorus B) germanium C) selenium D) tellurium E) none of these. C) selenium.
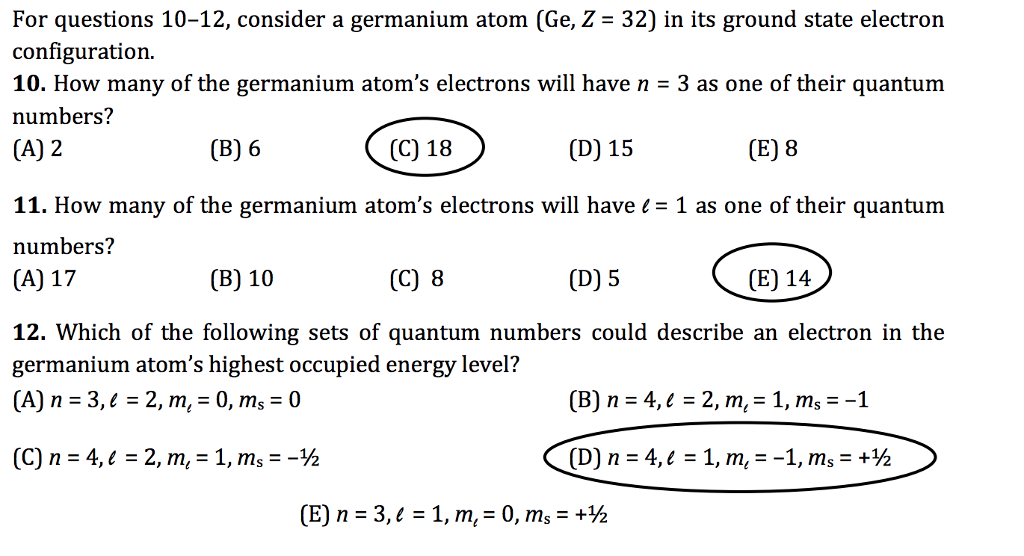
1. (30 pts) For germanium (Ge) atom: 1) Write the shorthand ground state electron configuration and orbital diagram for Ge. Indicate the magnetic property (diamagnetic or paramagnetic) of the atom. 2) Write a set of quantum numbers (n. l, m, m.) for one of the germanium valence electrons. 3) Based on the above orbital diagram, predict one ...

Solved Ques Tion 12 The Electron Configuration Of Gemanium Ge Is 152 2s22p6 3s2 3p6 4s2 4d104p2 0 152 2s2 2p6 3s2 3p6 4s2 3d104p2 0 1s2 2s2 7p6 3s2 2p6 452 4p2 2 Kr 5524d104p2
Orbital diagram of Germanium (Ge) 33: Orbital diagram of Arsenic (As) 34: Orbital diagram of Selenium (Se) 35: Orbital diagram of Bromine (Br) 36: Orbital diagram of Krypton (Kr) 37: Orbital diagram of Rubidium (Rb) 38: Orbital diagram of Strontium (Sr) 39: Orbital diagram of Yttrium (Y) 40: Orbital diagram of Zirconium (Zr) 41: Orbital diagram ...
The orbital structure for germanium is 2-8-18-4. You can understand why there are similarities to silicon and tin when you see their orbital structures are 2-8-4 and 2-8-18-18-4 respectively. Watch for the 4. Because it has traits of elements like silicon and tin (just underneath), it is used by industry as a semiconductor material. ...
The fourth orbital in this subshell lies along the X and Y axes and is called the 3d x 2-y 2 orbital. Most of the space occupied by the fifth orbital lies along the Z axis and this orbital is called the 3d z 2 orbital. The number of orbitals in a shell is the square of the principal quantum number: 1 2 = 1, 2 2 = 4, 3 2 = 9.
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
Ge (Germanium) is an element with position number 32 in the periodic table. Located in the IV period. Melting point: 937.4 ℃. Density: 5.32 g/cm 3 . Electronic configuration of the Germanium atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 2.
Electron Notation is: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2 Noble Gas notation is: [Ar] 4s2 3d10 4p2 Orbital Notation would be with the up & down arrow in boxes for each orbital.

Electron Configuration Orbital Notation And Quantum Mechanical Model Of Atom By Elena Man Ppt Download
Germanium (Ge) has an atomic mass of 32. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.
0 Comments. on Orbital Diagram For Germanium. orbital. Because an electron can have either one of two spins, any orbital can hold a maximum of four . The orbital diagram for germanium is. 1s. 2s. 2p. 3s. Oxidation States, +4,2. Electrons Per Shell, 2 8 18 4. Electron Configuration, [Ar] 3d10 4s2 4p2. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p2.
Germanium (Ge) is located in the fourth row, group 14 of the periodic table, and has an atomic number of 32. This implies that the neutral Ge atom's electron configuration must account for 32 electrons. So, "Ge": 1s^(2)2s^(2)2p^(6)3s^(2)3p^(6)4s^(2)3d^(10)4p^(2) An alternative way of writing the electron configuration for Ge is by using the noble gas shorthand notation.
1. The Pauli principle: No more than two electrons can occupy a given orbital. If there are two electrons in an orbital, their spins must be paired (one must have m s = 1 2 and the other, m s = − 1 2). 2. The aufbau (building-up) principle: When electrons are filled in to orbitals in an atom, the orbitals with lower energy are filled first.

Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes
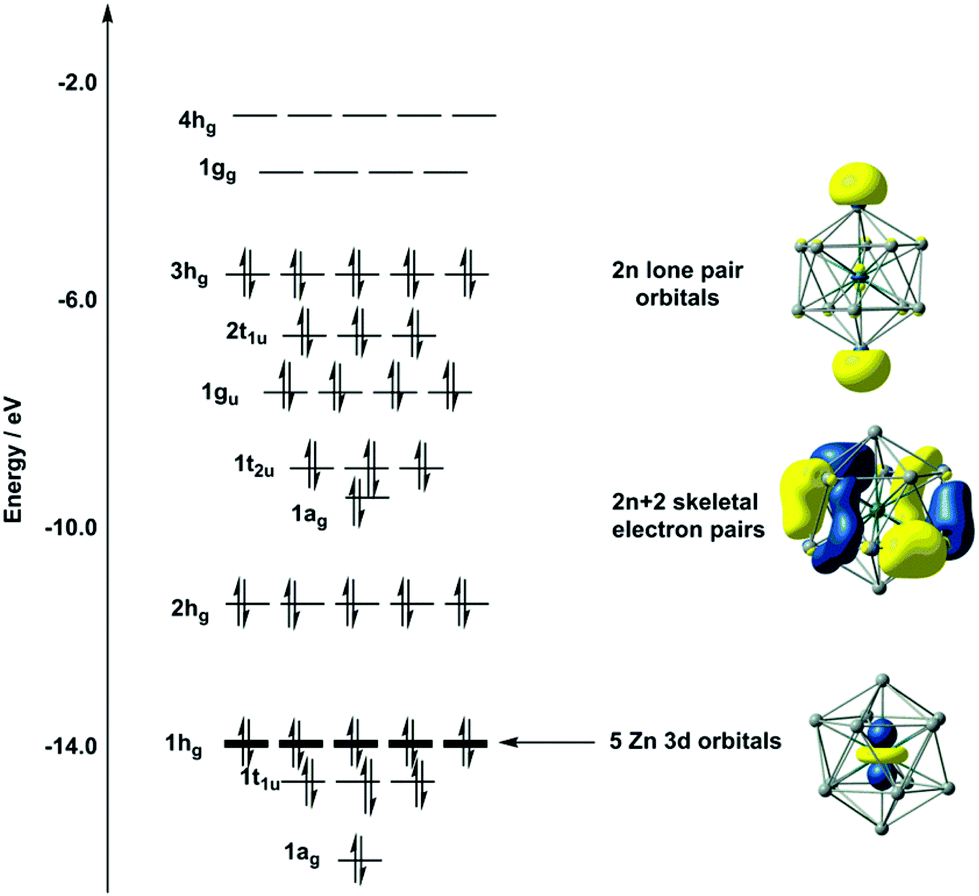
On The Structural Landscape In Endohedral Silicon And Germanium Clusters M Si 12 And M Ge 12 Dalton Transactions Rsc Publishing Doi 10 1039 C4dt03573a

See The Electron Configuration Diagrams For Atoms Of The Elements Electron Configuration Atom Diagram Element Chemistry
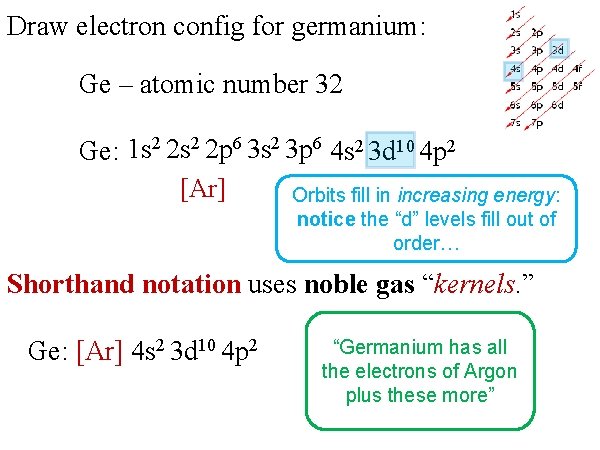

















0 Response to "41 orbital diagram for germanium"
Post a Comment