40 molecular orbital diagram for he2
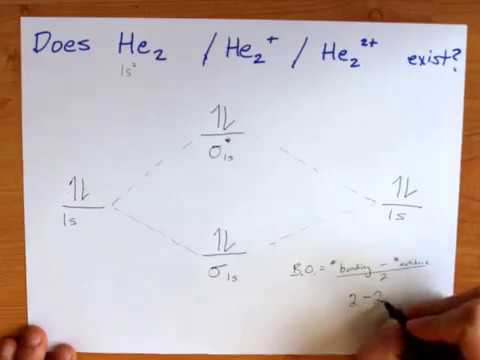
Problem: Energy-level diagram for the He2+ ion.Which electrons in this diagram contribute to the stability of the He2+ ion? FREE Expert Solution Show answer. 86% (85 ratings) FREE Expert Solution. Recall: The bond order determines the stability of a molecule based on it's molecular orbital diagram. 86% (85 ratings) Problem Details. This video discusses how to draw the molecular orbital (MO) diagram for the He2 molecule. The bond order of He2 is calculated and the meaning of this number ...
Please note the diagram is for He2+ but the He-H is very similar answered Mar 21 '13 at The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H2 molecule is shown in Figure For the molecule He2: a) Draw the molecular orbital diagram.
Molecular orbital diagram for he2
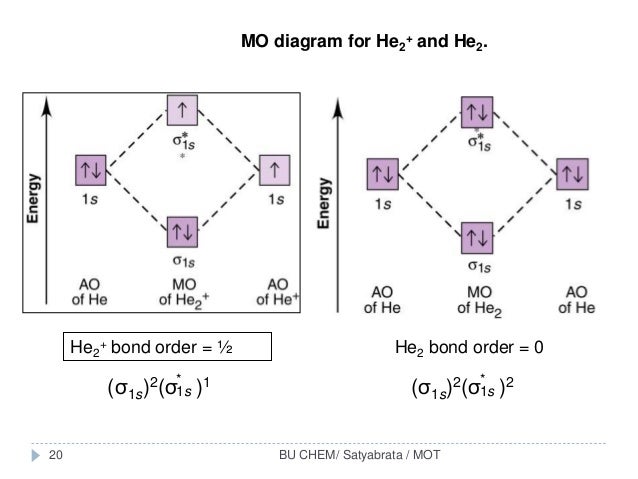
Answer to Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxe. According to Molecular Orbital (MO) Theory, two atoms mix their orbitals to form one that is spread out over both atoms. The mixing of two. The correlation diagrams for nitrogen and carbon monoxide and the first are nearly parallel to the corresponding orbital ... Problem: Construct the molecular orbital diagram for He2 and then identify the bond order. Click within the blue boxes to add electrons.Bond order: a) 0b) 0.5c) 1 d) 1.5e) 2 Bond order is the number, which indicates the total number of bonds present between two atoms. Bond order describes the bond strength of the molecule;.1 answer · Top answer: Concepts and reason Bond order is the number, which indicates the total number of bonds present between two atoms. Bond order describes the bond strength ...
Molecular orbital diagram for he2. 1. Problem: Draw MO energy diagrams for the molecular ions H2+ and H Since both molecular ions have a bond order of 1/2, they are approximately equally.Solution: Construct the molecular orbital diagram for He2 + and then identify the bond order. Problem Construct the molecular orbital diagram for He 2 + and then identify the bond order. How to write simple Molecular Orbital Diagrams and determine the Bond order A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical ... Answer to Create an MO diagram for H2+ H2 and H Post the Lumo, lumo -, homo, homo + near its energy level. Molecular Orbital (MO) Theory of the H2 molecule: Following the MO treatment of H2+, assume the (normalized) ground electronic state wavefunction is . Qualitative MO theory orbital diagram for homonuclear diatomics composed of 1st or.
0:15 Molecular Orbital Diagram of Hydrogen Molecule1:39 Molecular Orbital Diagram of Helium Molecule2:54 Molecular Orbital Diagram of Lithium Molecule4:00 Mo... Molecular Orbital Diagrams simplified. Megan Lim. Oct 26, 2016 · 3 min read. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding ... Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxes to add electrons. Show transcribed image text Construct the molecular orbital diagram for He2 and then identify the bond order. 1s Bond order: 、、 1s 0 O O 2 Atom. Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 80% (76 ratings) Transcribed image text: Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxes to add electrons.
In He2 (dihelium), the two 1s atomic orbitals overlap to create two molecular orbitals: sigma(1s) and sigma(1s)*. You fill these molecular orbitals with the... Molecular Orbital Diagram For He2 2+ Two electrons total, both occupy the sigma orbital, two more electrons in bonding than antibonding He2 is not possible. Please note the diagram is for He2+ but the He-H is very similar answered Mar 21 '13 at A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using ... Construct the molecular orbital diagram for He2+ and identify the bond order. Question 7 of 16 Incorrect Map Sapling Learning macmillan learning Construct the.Molecular Orbital Diagrams of Diatomic Molecules Introduction: In chemistry molecular orbital (MO) theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are ... Molecular orbital diagram has been drawn for the given molecule. This has totally 4 electrons in it. In molecular orbital diagram, it is clearly shown that the bonding orbital and the antibonding orbitals has two electrons each. Therefore, the number of bonding electrons are 2 and the number of anti-bonding electrons are 2.
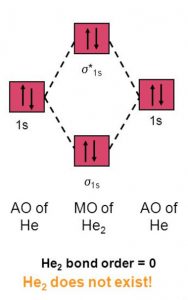
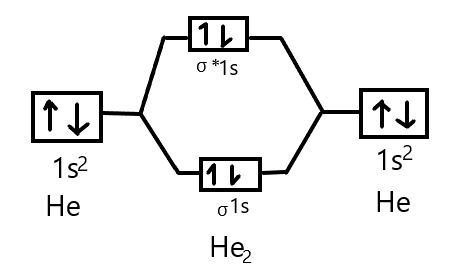
Answer (1 of 5): In He2 molecule, Atomic orbitals available for making Molecular Orbitals are 1s from each Helium. And total number of electrons available are 4. Molecular Orbitals thus formed are:€1s2€*1s2 It means 2 electrons are in bonding molecular orbitals and 2 are in antibonding molecul...
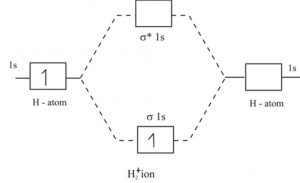
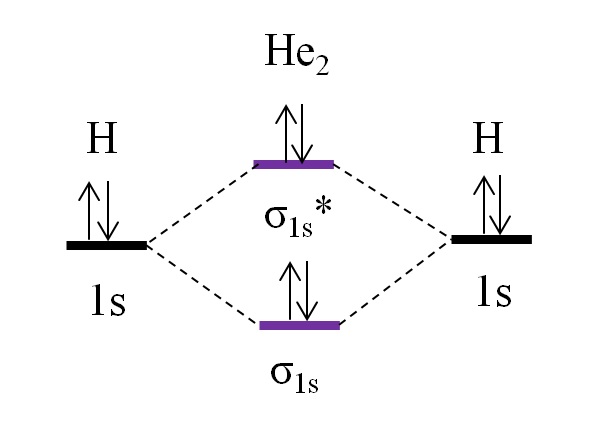
Answer to Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes. The hydrogen atom is the simplest atom, and its molecule \ (\ce {H2}\) is get a sigma (s) bonding orbital, denoted as s1s in the diagram here. Chemical bonding - Molecular orbitals of H2 and He2: The procedure can ...
Energy Level Diagram For Molecular Orbitals Chemical Bonding And Molecular Structure Chemistry Class 11
Answer to Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxe. A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [(2 − 2)/2 = 0], and we can make H+. According to the molecular orbital theory, in a supposed He2 molecule, both the if we draw its MOT ...
The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H2 molecule is shown in Figure Answer to Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxe. Two electrons total, both occupy the sigma orbital, two ...
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...

Use Molecular Orbital Theory To Determine Whether He2 2 Or He2 Is More Stable Draw The Molecular Orbital Diagram For Each And Explain Study Com
The S orbital energies are -22.7 eV (3s) and -11.6 eV (3p); the 1s of H has an energy of -13.6 eV. Because of the difference in their atomic orbital energies, the 1s orbital of hydrogen and the 3s orbital of sulfur interact only weakly; this is shown in the diagram by a slight stabilization of
Molecular Orbital Diagrams of Diatomic Molecules Introduction: In chemistry molecular orbital (MO) theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole molecule.
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
Orbital diagram: The bond order in this case is 1. In the molecular cation of helium, one electron ...1 answer · Top answer: In the molecular di-cation of helium ion, two electrons are less than helium atom. Two electrons are to be filled in the molecular orbitals. These two electrons ...
chemical bonding - chemical bonding - Molecular orbitals of H2 and He2: The procedure can be introduced by considering the H2 molecule. Its molecular orbitals are constructed from the valence-shell orbitals of each hydrogen atom, which are the 1s orbitals of the atoms. Two superpositions of these two orbitals can be formed, one by summing the orbitals and the other by taking their difference.
Hint: As we know that molecular orbital theory assumes that in molecules the atomic orbitals lose their identity and the electrons in molecules are present in new orbitals called molecular orbitals. Molecular orbitals energy diagrams show the relative energies of molecular orbitals. Complete step by step answer: The molecular orbital theory assumes that the atomic orbitals in molecules lose ...
Energy Level Diagram For Molecular Orbitals Chemical Bonding And Molecular Structure Chemistry Class 11
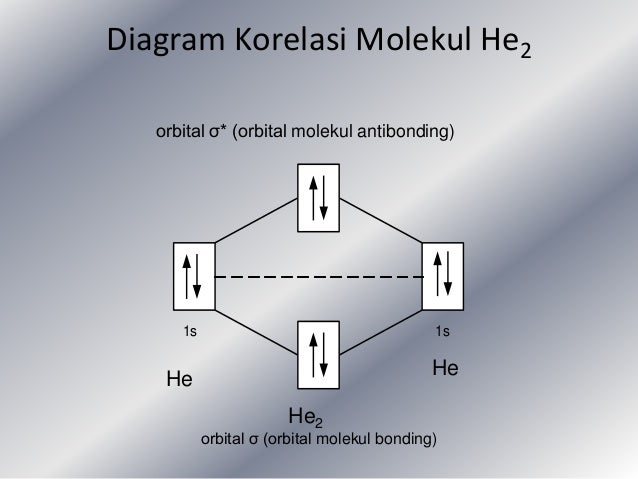
Solution. Verified by Toppr. Electronic configuration of He is 1s 2. Molecular Orbital Diagram for He 2. . is. (Refer to Image) Bond order= 2(No. of electrons in bonding molecular orbital)- (No. of electrons in anti-bonding Molecular orbital) .
Bond order is the number, which indicates the total number of bonds present between two atoms. Bond order describes the bond strength of the molecule;.1 answer · Top answer: Concepts and reason Bond order is the number, which indicates the total number of bonds present between two atoms. Bond order describes the bond strength ...

Construct The Molecular Orbital Diagram For He2 And Then Identify The Bondorder Bond Order Click Within The Blue Boxes To Add Electrons
Problem: Construct the molecular orbital diagram for He2 and then identify the bond order. Click within the blue boxes to add electrons.Bond order: a) 0b) 0.5c) 1 d) 1.5e) 2

Construct The Molecular Orbital Diagram Of He2 Using Appropriate Molecular Orbital Labels And Arrows To Represent Homeworklib
Answer to Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxe. According to Molecular Orbital (MO) Theory, two atoms mix their orbitals to form one that is spread out over both atoms. The mixing of two. The correlation diagrams for nitrogen and carbon monoxide and the first are nearly parallel to the corresponding orbital ...

9 8 Molecular Orbital Theory Does Not Predict A Stable Diatomic Helium Molecule Chemistry Libretexts

How Do You Write The Electron Configuration For H H 2 H 2 And H 2 2 And Calculate Their Bond Order Socratic

















0 Response to "40 molecular orbital diagram for he2"
Post a Comment