40 democritus atom model diagram
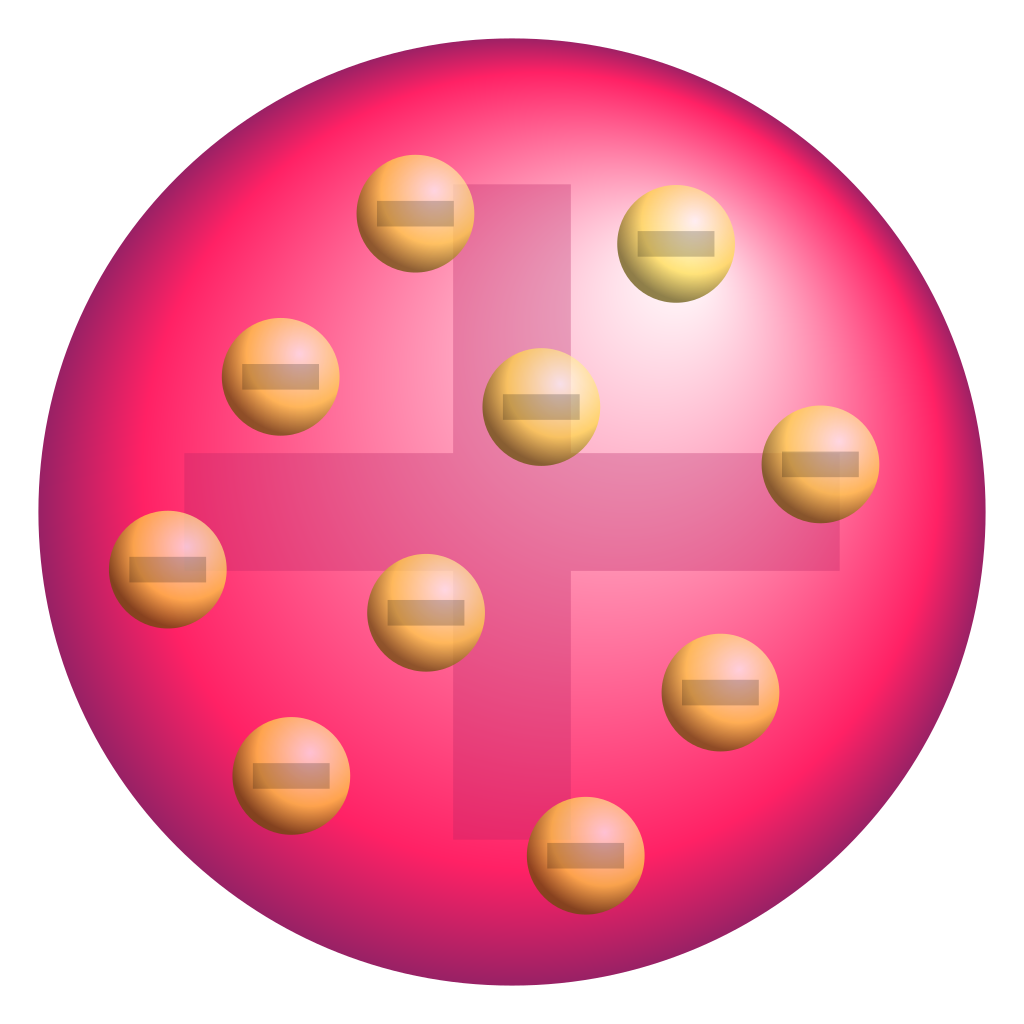
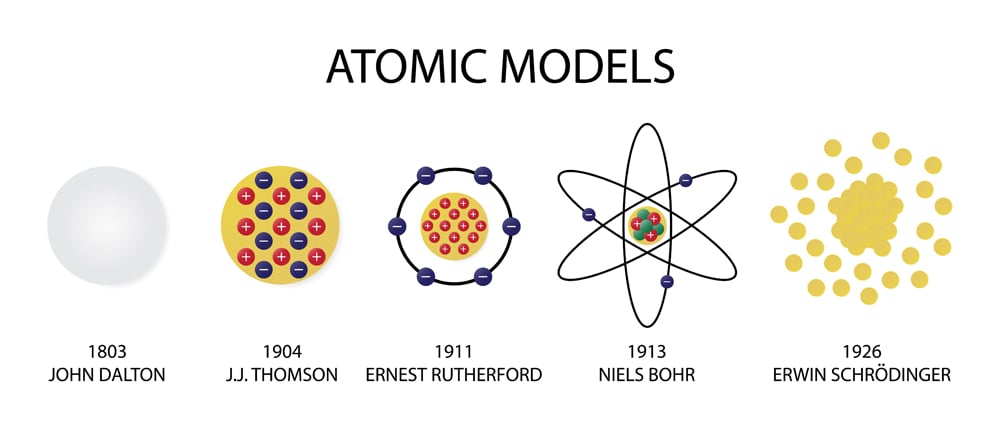
Around 450 B.C., the Greek philosopher Democritus introduced the idea of the atom. He proposed the plum pudding model of the atom. In this model, negative electrons are scattered throughout a "sea" of positive charge. In 1911, Ernest Rutherford discovered the nucleus. Ancient History of the Atom. Democritus (460-370 BC), a Greek philosopher, was the first person to use the word atom or atomos (in Greek), which means indivisible or unbreakable, to describe the smallest particle of any substance. He believed that atoms were too small to be seen.
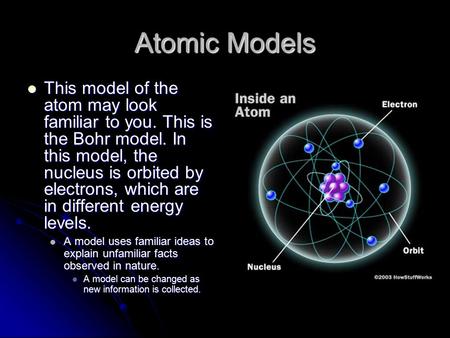
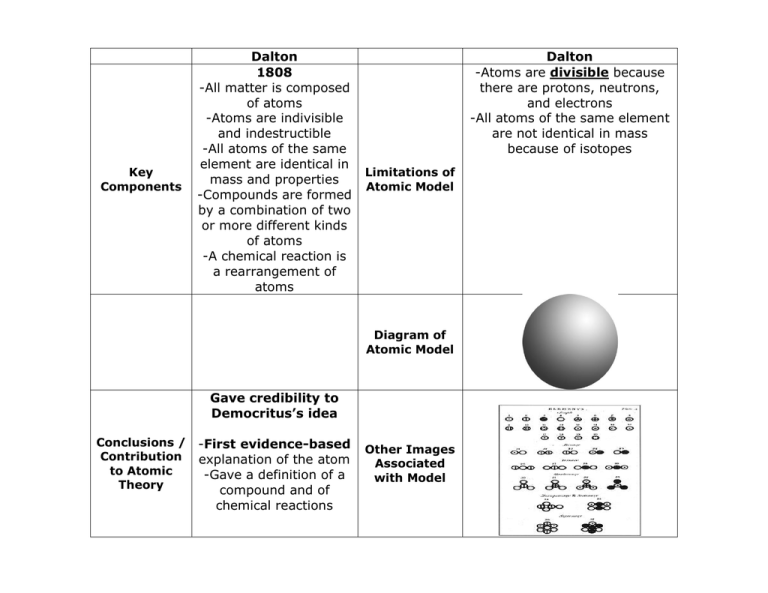
This model has some good ideas in it, but overall it has some problems. The key (and not incorrect points) of this model are: The atom is made of protons, neutrons and electrons. Most of the space ...
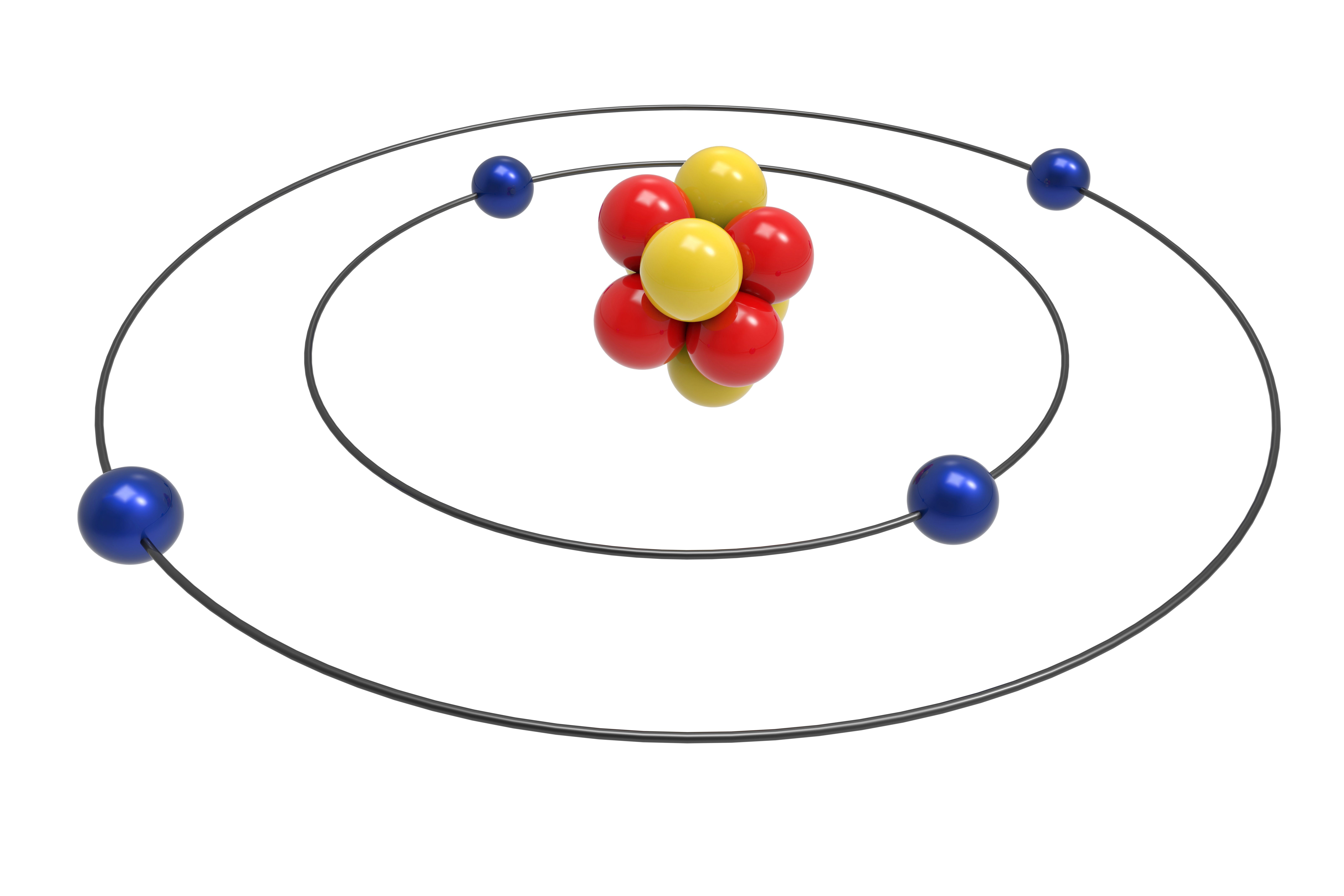
Democritus atom model diagram
Democritus Atom Model Diagram. Democritus was an Ancient Greek pre-Socratic philosopher primarily remembered today for his formulation of an atomic. This is due to his theory of universe that is made up of tiny "atoms", which bears .. Democritus' model of an atom was one of an intert solid that. In this lesson, we will review the ... Democritus Atom Model Diagram. The idea of atoms was invented by two Greek philosophers, Democritus and Leucippus in the fifth century BC. The Greek word ατoμoν (atom) means indivisible. B.C. - Democritus thought matter could not be John Dalton. • -Dalton proposed a modern atomic model Bohr - Rutherford diagrams. The atomic model of Democritus was the first to introduce the idea that matter consists of indivisible basic elements called "atoms". In fact, the word atom means indivisible. Democritus was a Greek thinker who lived between 460 BC and 370 BC. He was the father of atomism and a disciple of other Greek philosophers such as Leucippus and Anaxagoras. Democritus reaches …
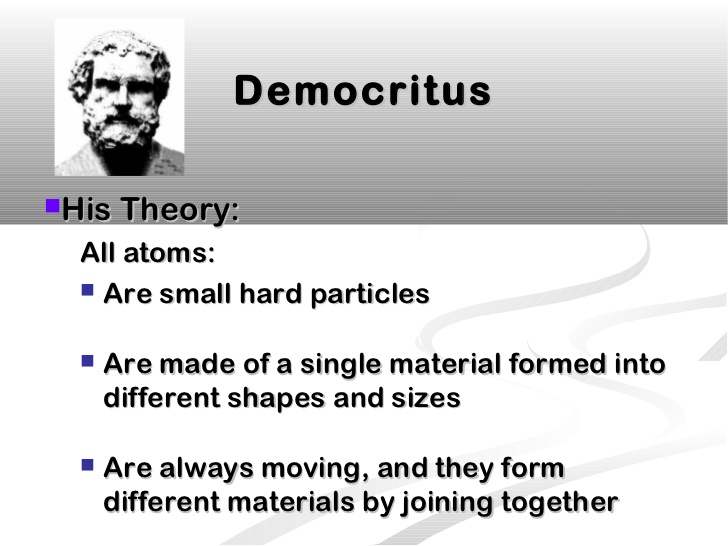
Democritus atom model diagram. Atomic model of Democritus. For many years, man has wondered how matter is formed . Matter, which is a fundamental aspect in the area of chemistry , is composed of small particles which we all know by the name of atoms , which can have specific behavior and properties . . Many atomic models have been developed over time, and they are usually named after who created them or who discovered them. Atom theory according to Democritus: Atom is the smallest form of elements that form reality. It can't be seen by plain view because of the extremely small size. Atom has no quality, but it has quantity and mass number. Atom can be differed by its shape and portion. The number of atoms that formed reality is infinite. The key difference between Democritus and Dalton atomic theory is that the Democritus atomic theory is an ancient theory that scientists later refined and elaborated whereas Dalton atomic theory is a comparatively modern, scientific theory that we cannot discard due its important statements.. Atomic theory is the scientific theory that describes the nature of matter by means of discrete units ... The Rutherford model of the atom is one of the most popular representations of the atom. Explore the definition, diagram, development, and problems of the Rutherford model, as well as the ...
A depiction of the atomic structure of the helium atom. Credit: Wikipedia/Creative Commons. This became the basis as Dalton's Law (aka. Dalton's law of partial pressures), which stated that in ... Information Atomic Model Analogy In 1897, the English scientist named J.J. Thomson provided the first hint that an atom is made of even smaller particles. He discovered the presence of a negative particle in the atom - the electron. He proposed a model of the atom that is sometimes called the "Plum Pudding" model. Learn democritus atomic model with free interactive flashcards. Choose from 500 different sets of democritus atomic model flashcards on Quizlet. Each atom (of a different substance) is different in size, weight and shape. Timeline: 1800's Scientist: John Dalton John Dalton was the first to adapt Democritus' theory into the first modern atomic model. JOHN DALTON'S ATOMIC MODEL: 1. All matter consists of tiny particles called atoms 2. Atoms are indestructible and unchangeable 3.
1. Democritus was not able to describe atomic model in detail. On his theory, Democritus only stated that atoms are in the solid form in the void sphare. We can not describe the internal structure of the atom itself. We now know that Atoms consist of 3 parts which are proton, neutron and electron. 2. Rockwell Portable Baandsaw Model 725 Wiring Diagram; Labeled Starfish Diagram; Strawman Diagram Visio; Predator Generator 69671 Wiring Diagram; K40 Radar Wiring Diagram; 2006 Gmc C7500 Wiring Diagram; Mobility Svm Wiring Diagram; Democritus Atom Model Diagram; 4 Pole Wireless Mic Headphone Jack Mini Xlr Wiring Diagram; 94 Zzr 1100 Bad Ground ... Democritus and the Atom Even though Democritus was the first to use the word atom he wasn't recognized for it and never had a atomic model or theory. Democritus' idea and use of the word "Atom" was the first step to building the foundation of chemistry with the atom thousands of years later! 900 seconds. Q. This was the first model of the atom ever proposed. It was simple and described atoms as tiny spheres that could not be broken down into smaller pieces. answer choices. Democritus's model of the atom. The "Plum Pudding Model" of the atom. The "Rutherford Model" of the atom. The "Solar System Model" of the atom.
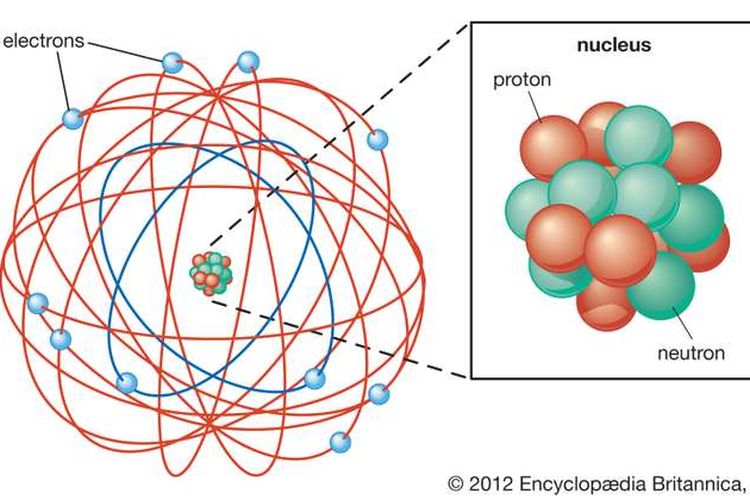
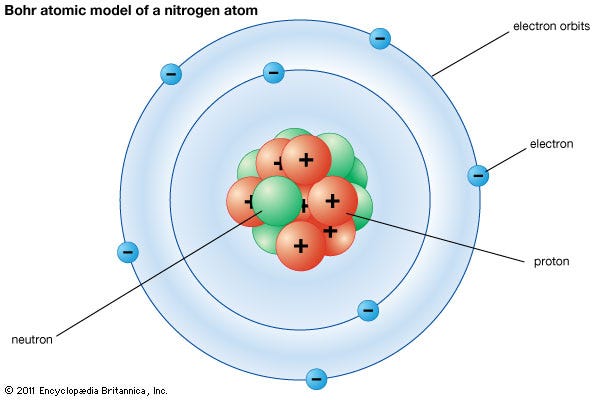
Key Takeaways: Model of the Atom. An atom is a building block of matter that cannot be broken apart using any chemical means. Nuclear reactions can alter atoms. The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). Protons and neutrons form the atomic nucleus.
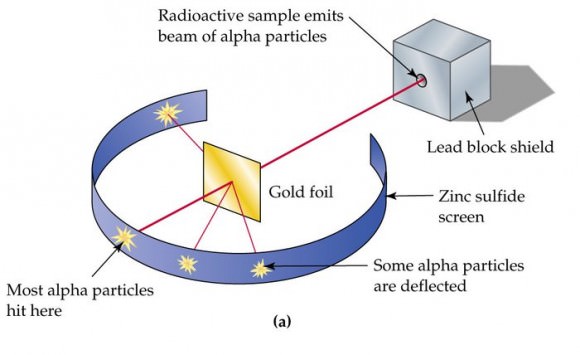
The nucleus is very small compared to the size of atom and the entire mass of an atom is centered in the nucleus. This conclusion helped him propose 'Rutherford's Atomic Model'. According to his atom diagram, the atom has a small, positively charged nucleus in center. This nucleus carries the entire mass of the atom.
Answer (1 of 4): Atoms exist! …that's basically it. Democritus was first and foremost a philosopher, and while he made some scientific contributions to atomic theory, he was much more concerned with what it meant for the human condition. Mainly, that atoms helped explain his concept of determin...
Democritus atom diagram. Wiki User. ∙ 2008-10-10 16:10:25. See Answer. Best Answer. Copy. if i knew i wouldn't be in here trying to look for an answer or whatever that is, don't you think im ...
Democritus' atom: a long-forgotten model. For Aristotle the atomism of Democritus contradicted the concept of substance, in which the proportion of the elements (earth, air, water and fire) had to be maintained at all costs, no matter how small the fraction of it was. The substance for Aristotle is intrinsically continuous.
Democritus and Aristotle greatest difference where their views with atoms.Democritus believed that the atom did exist and was the smallest unit of matter. Aristotle believe that there could be no the existence of the atom.Aristotle was incorrect , Democritus. they both developed their theory around 400 and 300 B.C in the era of ancient Greek ...
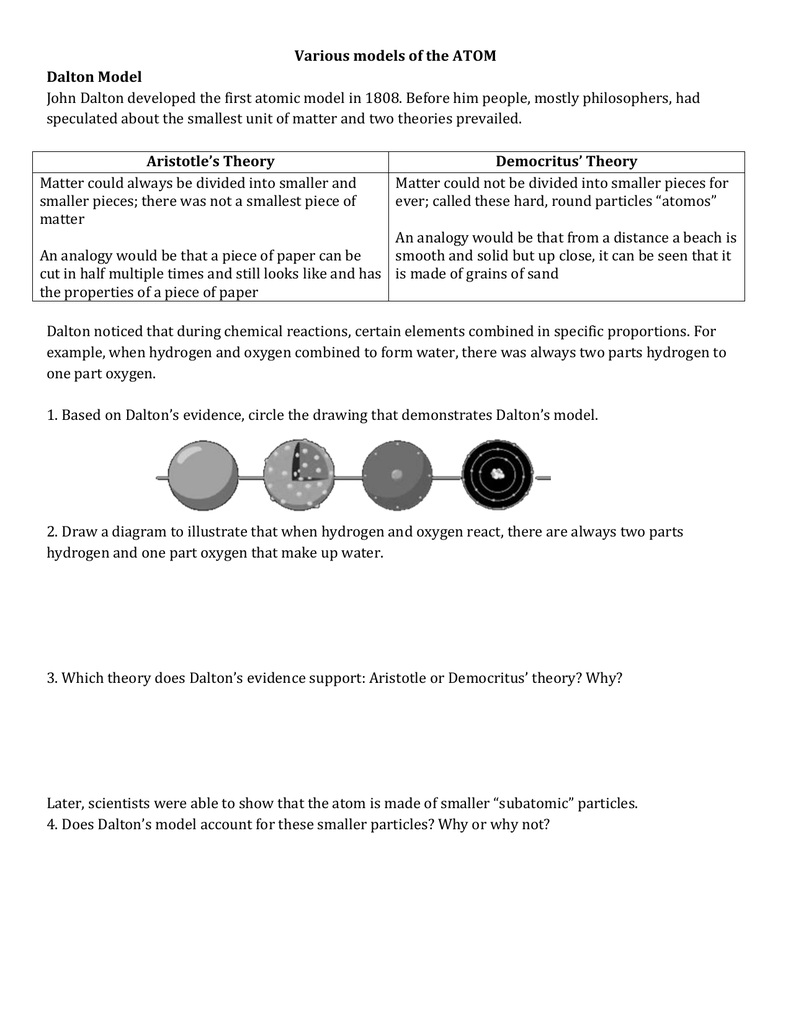
Put forward atomic model in: 1803 Nickname for his model: Billiard Ball Model Description of his model: Dalton was an English chemist and teacher who used experimental evidence to form the atomic theory of matter: All elements are composed (made up) of atoms. It is impossible to divide or destroy an atom. All atoms of the same element are alike.
Atomic Theory Png Democritus Atomic Theory John Dalton Atomic Theory Experiment Thomson Atomic Theory Model John Dalton Atomic Theory Model Name Atomic Theory Powerpoints Animated Atomic Theory Models Did Atomic Theory John Dalton Contribution To
Democritus is criticized by Aristotle for supposing that the sequence of colliding atoms has no beginning, and thus for not offering an explanation of the existence of atomic motion per se, even though the prior collision with another atom can account for the direction of each individual atomic motion (see O'Keefe 1996). Although the ancient ...
Democritus' model of an atom was one of an inert solid that interacted mechanically with other atoms. Credit: .science.edu.sg. However, Democritus is credited with illustrating and popularizing ...
Democritus was born in Abdera, around 460 B.C. Due to the fact that there was no technology, Democritus was unable to perform experiments; therefore, Democritus had no evidence of his theory, but it was proved to be somewhat close to what was discovered 2000 years later.
DEMOCRITUS. Lived from: 460-370 BC. Put forward atomic model in: 442 BC. Description of his model: Democritus's model stated that matter consists of invisible particles called atoms and a void (empty space). He stated that atoms are indestructible and unchangeable. Also that they are homogenous, meaning they have no internal structure.
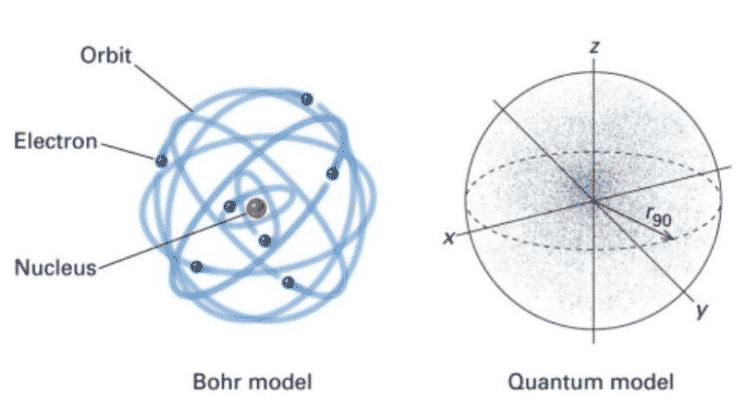
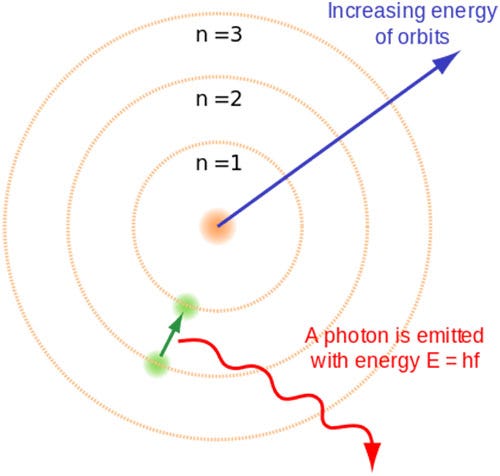
Democritus's _____7. Whose model determined that an atom's positive charge is concentrated in the atom's center? a. Rutherford's c. Democritus's ... Unlike the modern model of the atom, Bohr's model states that a. electrons move in set paths around the nucleus of an atom. ... Use the diagram to the right to answer question 1. _____1 ...
The atomic model of Democritus was the first to introduce the idea that matter consists of indivisible basic elements called "atoms". In fact, the word atom means indivisible. Democritus was a Greek thinker who lived between 460 BC and 370 BC. He was the father of atomism and a disciple of other Greek philosophers such as Leucippus and Anaxagoras. Democritus reaches …
Democritus Atom Model Diagram. The idea of atoms was invented by two Greek philosophers, Democritus and Leucippus in the fifth century BC. The Greek word ατoμoν (atom) means indivisible. B.C. - Democritus thought matter could not be John Dalton. • -Dalton proposed a modern atomic model Bohr - Rutherford diagrams.
Democritus Atom Model Diagram. Democritus was an Ancient Greek pre-Socratic philosopher primarily remembered today for his formulation of an atomic. This is due to his theory of universe that is made up of tiny "atoms", which bears .. Democritus' model of an atom was one of an intert solid that. In this lesson, we will review the ...


















0 Response to "40 democritus atom model diagram"
Post a Comment