38 iodine electron dot diagram
Lewis Structure Lewis structure is the representation of the electrons of the molecules. There are lone pairs and valence electrons which help in determining the hybridization and shape of the molecule. As there are molecules of Iodine, one molecule of Iodine will be in the centre. Also, iodine is in the seventh group of the periodic table and ... Hydrogen and Iodine are both non-metals, so they form a COVALENT bond and SHARE electrons to complete their outer shells. Hydrogen shares one electron with i...
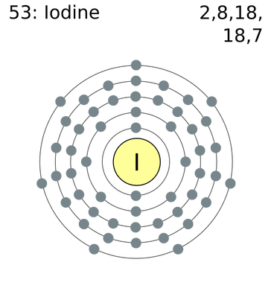
Comprehensive information for the element Iodine - I is provided by this page including scores of properties, element names in many languages, most known nuclides and technical terms are linked to their definitions. ... Atomic Structure of Iodine. ... Electron Dot Model. Chemical Properties of Iodine. Electrochemical Equivalent: 4.7348g/amp-hr;
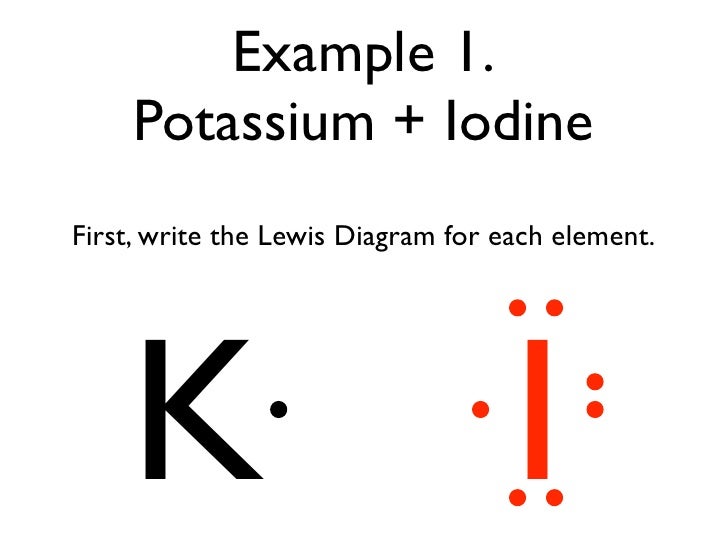
Iodine electron dot diagram
Iodine is a diatomic molecule so its molecules are paired as i2 iodine has 7 electrons in its outer shell so 1 electron is shared by each atom. To make the electron dot diagram you put the electron symbol and put a dot on one of the sides for. Chemical properties of iodine. The left diagram shows a Lewis dot structure of sodium with. Likewise, they can be used .. The left shows an iodine atom with one lone pair. When we write the . Comprehensive information for the element Iodine - I is provided by this page including scores of properties, Atomic Structure of Iodine Electron Dot Model . Cyanogen iodide may cause convulsions, paralysis and death from respiratory failure. It is a strong irritant and may cause burns to the eyes and skin if contacted. If cyanogen iodide is heated enough to undergo complete decomposition, it may releases toxic fumes of nitrogen oxides, cyanide and iodide. A fire may cause the release of poisonous gas.
Iodine electron dot diagram. Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at 114 degrees Celsius, and boils to a violet gas at 184 degrees Celsius.The element was discovered by the French chemist Bernard Courtois in 1811, and was named two years later ... Iodine Pentachloride, ICl5 Molecular Geometry & Polarity. ICl5 : First draw the Lewis dot structure: Electron geometry: octahedral. Hybridization: sp 3 d 2. Then construct the 3D geometry using VSEPR rules: Decision: The molecular geometry of ICl 5 is square pyramid with an asymmetric electron region distribution. Therefore this molecule is polar. NI3 lewis structure contains three N-I bonds, nitrogen in center position whereas all three iodine atoms are at the terminal position. There is only one lone pair present on the central atom in the NI3 lewis structure. Each iodine atom at the surrounding position contains three lone pairs and is connected to the central atom with a single bond. Iodine Dot Diagram Lewis Structure Black Png Download 900900 Free Transparent. Iodine Dot Diagram Electron Dot Diagram For I2 Wiring Diagrams Interval. Iodine Dot Diagram Lewis Dot Diagram For Several Molecular Models A E And For Bulk Au. Iodine Dot Diagram Iodine Pentafluoride Molecular Geometry Blog.
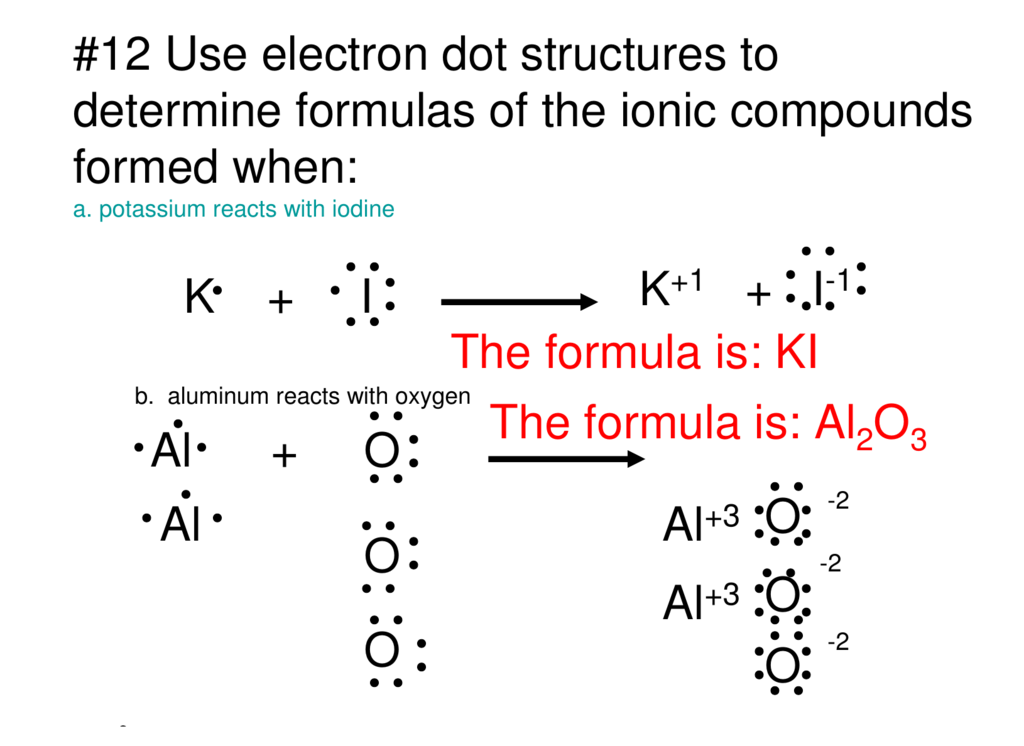
Comprehensive information for the element Iodine - I is provided by this page including scores of properties, Atomic Structure of Iodine Electron Dot Model . The left diagram shows a Lewis dot structure of sodium with. Likewise, they can be used .. The left shows an iodine atom with one lone pair. When we write the . A step-by-step explanation of how to draw the I2 Lewis Dot Structure (Iodine Gas).For the I2 structure use the periodic table to find the total number of val... Hence, the Lewis structure of iodine trichloride would be: We can observe that every chlorine atom is surrounded by eight electrons but a central atom, iodine, is surrounded by ten electrons. It is one of the exceptions of the octet rule, i.e., the elements of the third period or beyond the third period of the periodic table have 3d electrons ... Iodine Electron Dot Diagram. which is the correct electron dot diagram for iodine this site might help you re which is the correct electron dot diagram for iodine plz with a pic or if u cnt wit o a pic jus tell me how it electron dot diagram for iodine imageresizertool electron dot diagram for iodine moreover new website periodic table as well as watch along with 9 further also 140 explain ...
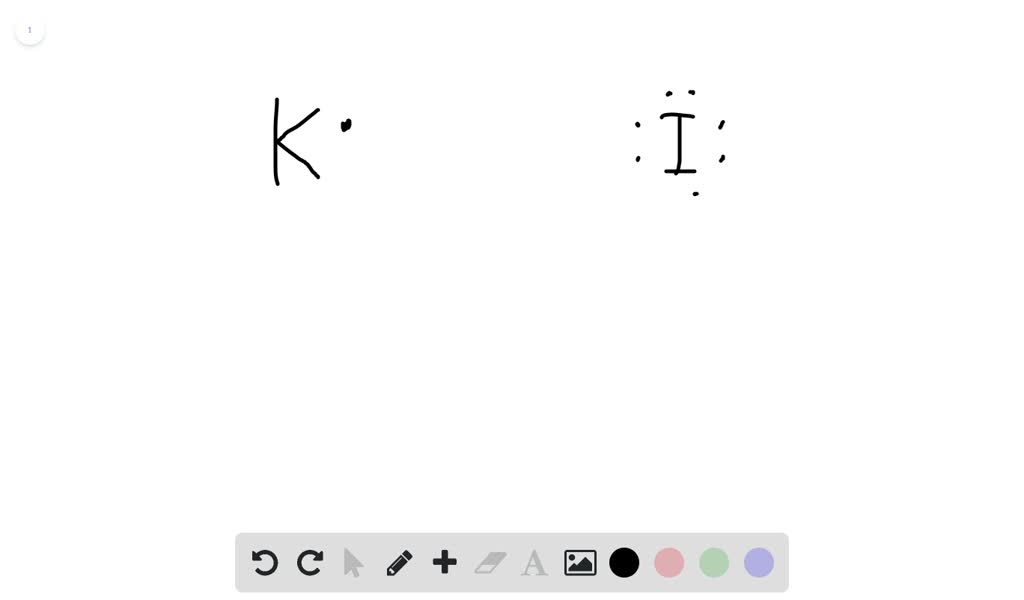
In a Lewis structure, atoms that are bonded covalently are represented by a single line joining the two atoms, which are represented by the element's chemical symbol. Covalent bonds occur mainly in diatomic molecules, such as hydrogen, nitrogen, fluorine, chlorine, bromine, iodine, and astatine. Electron Dot Diagram for Iodine. which is the correct electron dot diagram for iodine plz with a pic or if u cnt wit o a pic jus tell me how it looks like electron dot diagram for iodine imageresizertool electron dot diagram for iodine moreover new website periodic table as well as watch along with 9 further also 140 explain using dot and cross in Hydrogen Iodide (HI) Lewis Structure. Lewis structure of Hydrogen iodide (HI) contains only one H-Br bond. There are no charges on atoms in HI lewis structure because HI is a neutral molecule. There is three lone pairs on bromine atom in HI molecule. HI is a very easy lewis structure to draw due to its simplicity. Electron Dot Diagram For Iodine Luxury 58 Recent Lewis Dot Diagram Lewis dot diagram for potassium and iodine. Lewis dot diagram for iodine. Were going to draw the lewis structure for i2 iodine gas a very pretty purple gas. Posted on march 26 2019 by admin. Hence iodine exists as the diatomic i2 as shown.
Iodine monochloride, 99.998% trace metals basis. Q414607. Iodine monochloride solution, 1.0 M in methylene chloride. Iodine monochloride, 1M solution in dichloromethane, AcroSeal (R) Iodine monochloride, ACS reagent, 1.10+/-0.1 I/Cl ratio basis. Iodine monochloride, approx. 0.22N soln. in glacial acetic acid.

Iodine Electron Configuration Symbol Atomic Number Atomic Mass Oxidation States Standard State Group Block Year Discovered
While the Lewis Structure provides an idea about the physical attributes of the compound, its representation is limited since it is a 2-dimensional model. It also does not reflect upon the molecular design, geometry, or the 3-dimensional representation of atoms. below are the steps to draw the lewis diagram of the Br2 molecule.
on Iodine Electron Dot Diagram. Comprehensive information for the element Iodine - I is provided by this page including scores of properties, Atomic Structure of Iodine Electron Dot Model . Lewis dot structures help predict molecular geometry. This example Total valence electrons to be "happy" = 1 iodine (8) + 3 chlorine (3 x 8).
For Iodine we have 7 valence electrons, and 7 for the Chlorine; total of 14 valence electrons for the ICl Lewis structure. We'll put the Iodine here, and the Chlorine right next to it. We have a total of 14 valence electrons. We'll put 2 between atoms to form the chemical bond, and we'll go around the outside.
Transcript: This is Dr. B. We're going to draw the Lewis structure for I2, Iodine gas, a very pretty purple gas. So let's start out. Iodine is in group 7 of the periodic table. That means it has 7 valence electrons, so we have 7. But we have two Iodine atoms, so we need to multiply that by two, giving us a total of 14 valence electrons.
Iodine is a naturally occurring element found in sea water and in certain rocks and sediments. There are non radioactive and radioactive forms of iodine. Iodine is used as a disinfectant for cleaning surfaces and storage containers and is used in skin soaps and bandages, and for purifying water. Iodine is also added to some table salt to ensure that all people in the United States have enough ...
Since Iodine (I) is below Period 3 on the periodic table it can hold more than 8 electrons. In the Lewis structure for ICl4- the Iodine atom has 12 valence electrons. Also note that you should put the ICl4- Lewis structure in brackets with as 1- on the outside to show that it is an ion with a negative one charge.
Lewis structure of iodine molecule contains only one I-I bond and each iodine atom has three lone pairs. It is very easy to draw the I 2 lewis structure. I 2 lewis structure. There is only a single bond between iodine atoms and three lone pairs on each iodine atoms. So, this lewis structure is a very simple.
Drawing the Lewis Structure for IF 5. Video: Drawing the Lewis Structure for IF 5. Iodine is below Period Two on the periodic table so it can have an expanded octet (hold more than eight valence electrons). In the Lewis structure for IF5 you'll need to put a total of 12 valence electrons on the Iodine atom in order to draw the Lewis structure.
ICl2- lewis structure contains one iodine atom at the middle position whereas two chlorine atoms at the surrounding position. There are three lone pairs present on the central atom of ICl2- lewis structure. Also, the iodine central atom in ICl2- lewis structure violates the octet as it is holding more than 8 electrons in its octet shell.

Lewis Dot Structures Lewis Dots Lewis Dot Structures Describe The Covalent Bonding In Molecules They Describe How The Valence Electrons Outermost S Ppt Download
Iodine fluoride has the molecular formula of IF3. It is composed of one iodine (I) and three fluoride (F) atoms. The Lewis dot structure for iodine fluoride is (the four eletrons above iodine ...
Cyanogen iodide may cause convulsions, paralysis and death from respiratory failure. It is a strong irritant and may cause burns to the eyes and skin if contacted. If cyanogen iodide is heated enough to undergo complete decomposition, it may releases toxic fumes of nitrogen oxides, cyanide and iodide. A fire may cause the release of poisonous gas.
The left diagram shows a Lewis dot structure of sodium with. Likewise, they can be used .. The left shows an iodine atom with one lone pair. When we write the . Comprehensive information for the element Iodine - I is provided by this page including scores of properties, Atomic Structure of Iodine Electron Dot Model .
Iodine is a diatomic molecule so its molecules are paired as i2 iodine has 7 electrons in its outer shell so 1 electron is shared by each atom. To make the electron dot diagram you put the electron symbol and put a dot on one of the sides for. Chemical properties of iodine.
When A Covalent Lewis Structure Is Drawing Using One Nitrogen N How Many Lodine Atoms Will Bond To The Nitrogen Quora




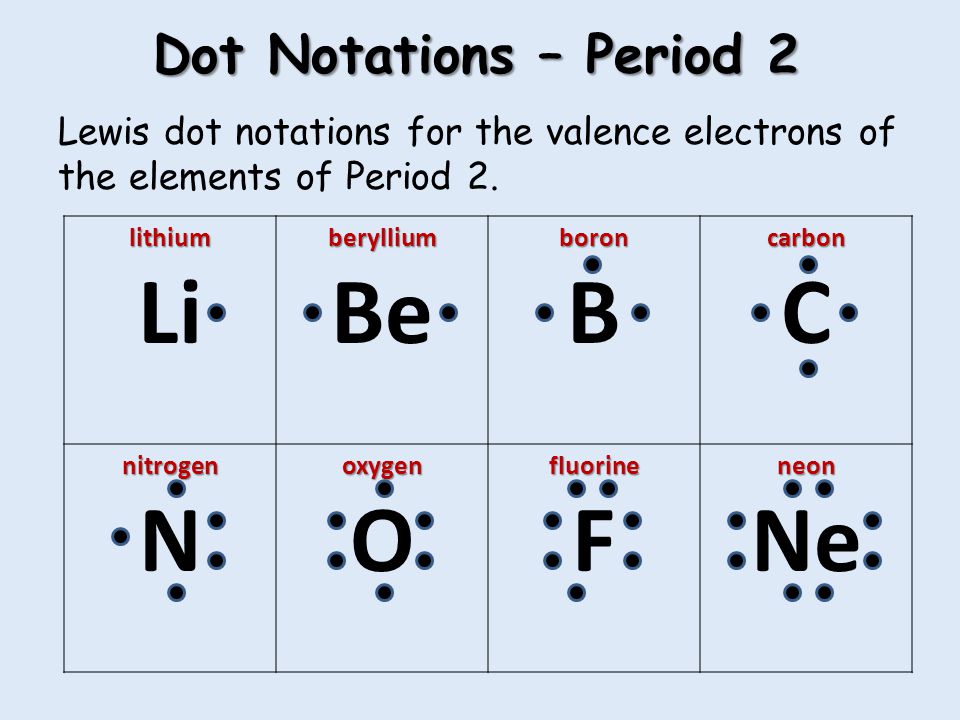





:max_bytes(150000):strip_icc()/lewis-fc84e3f1452e4aacb2fe023cfff2fa08.jpg)
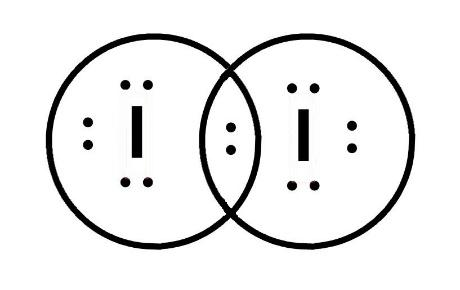











/ICl3_LD-56a12a2b3df78cf77268034c.png)
0 Response to "38 iodine electron dot diagram"
Post a Comment