38 mo diagram for hf
› fact › documentationAll Phase Diagrams - Polytechnique Montréal Click on a system to display the phase diagram. (NH4)2SO4-H2O FTfrtz (NH4) ... C-Hf SpMCBN: C-Hf-Mo_1673K SpMCBN: C-Hf-Mo_1973K SpMCBN: C-Hf-N_1423K SpMCBN: C-Hf-Nb ... topblogtenz.com › cyanide-cn-lewis-structureCN- lewis structure, molecular orbital diagram, and, bond order In this article, we will study the Cyanide (CN-) lewis structure, molecular orbital diagram(MO), its bond order, formal charges, and hybridization. Cyanide can be a colorless gas in the form of hydrogen cyanide, sodium cyanide, potassium cyanide, etc.
sites.google.com › site › catcalcphaseエリンガム図 / Ellingham diagram - Phase Diagram 金属やセラミックスの状態図、エンリンガム図などの情報を提供しています。 一部、不確かなものもありますので、自己 ...

Mo diagram for hf
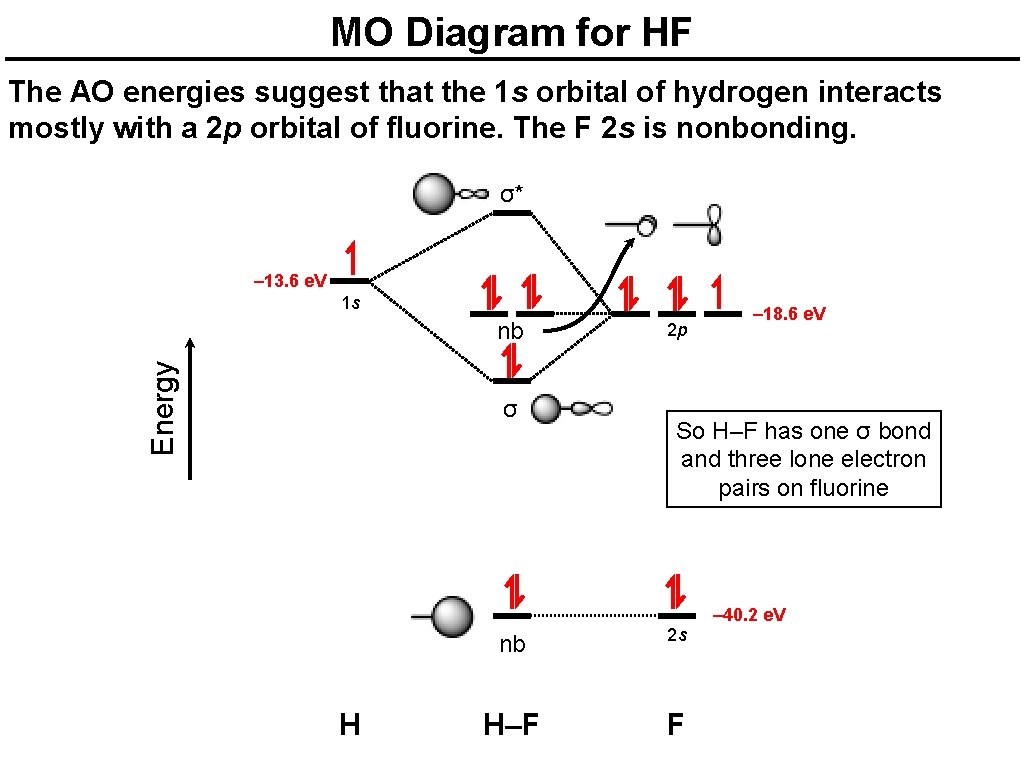
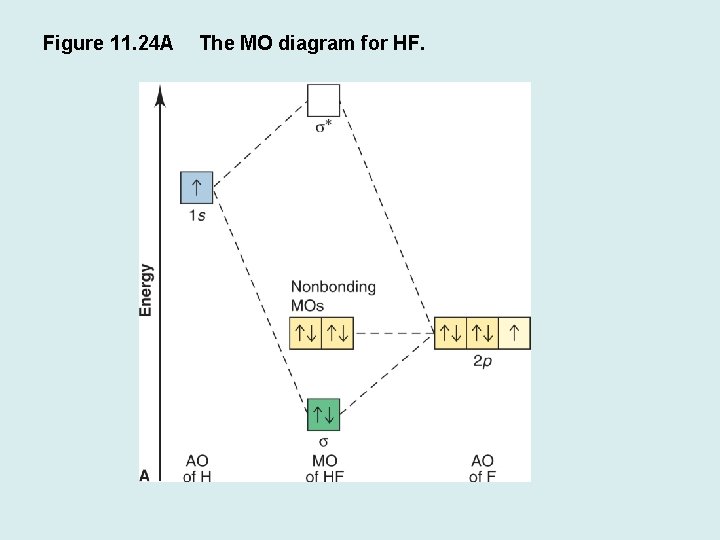
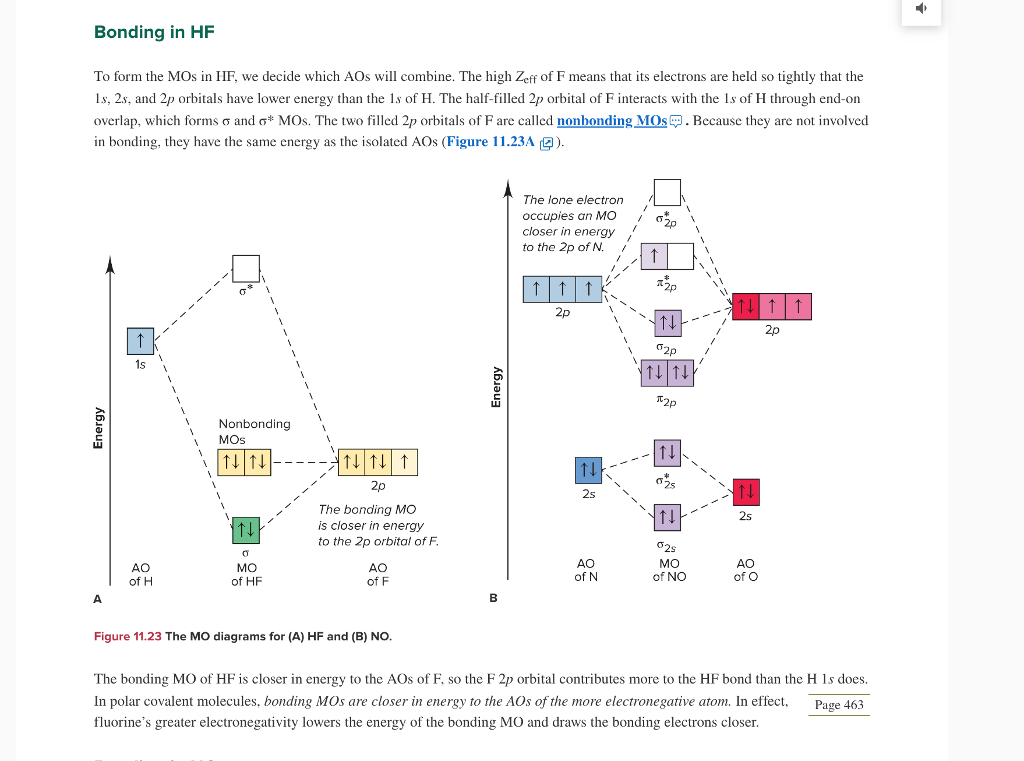
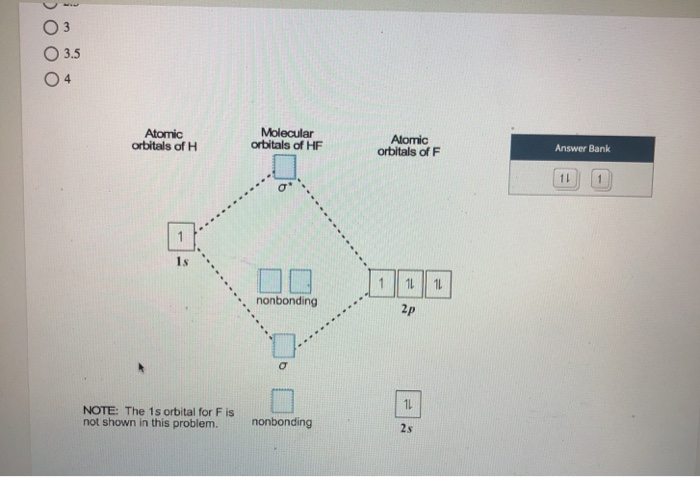
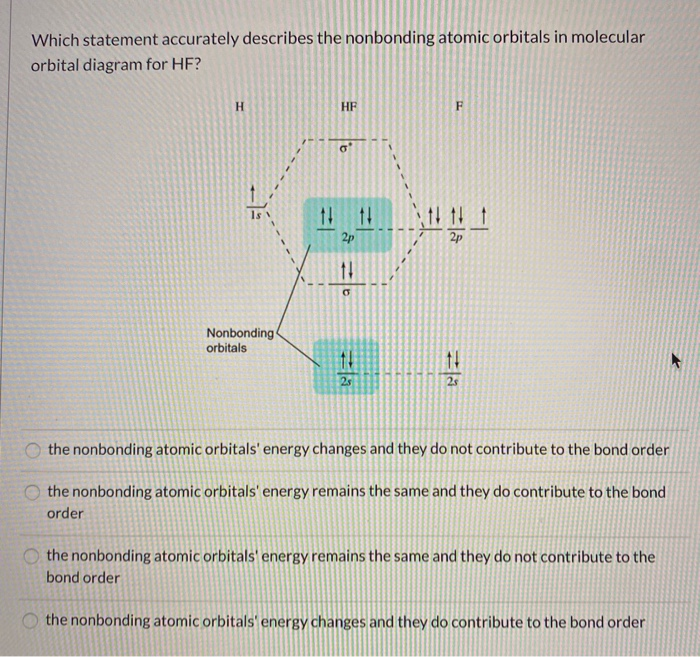
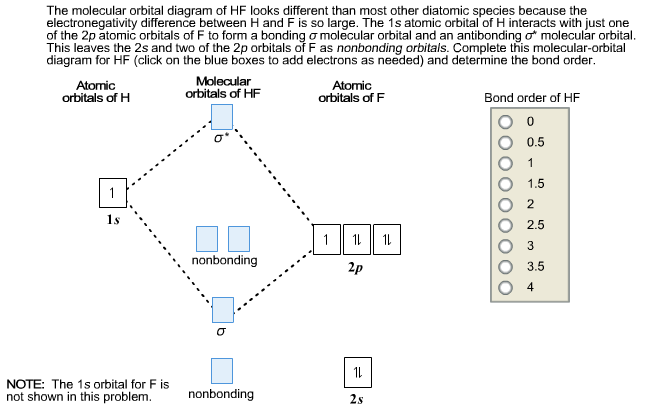
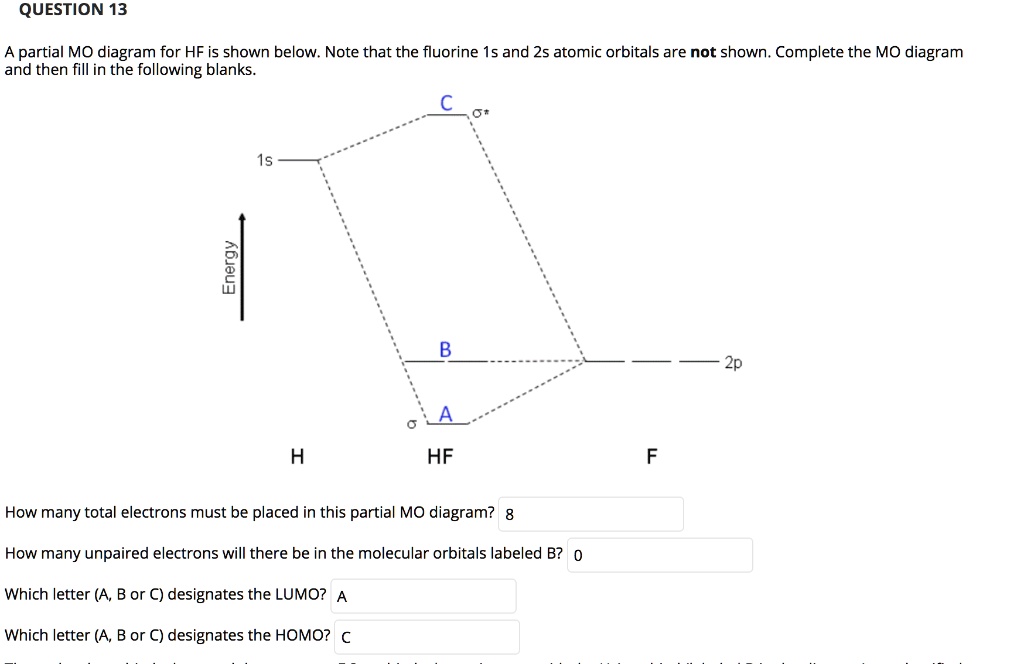
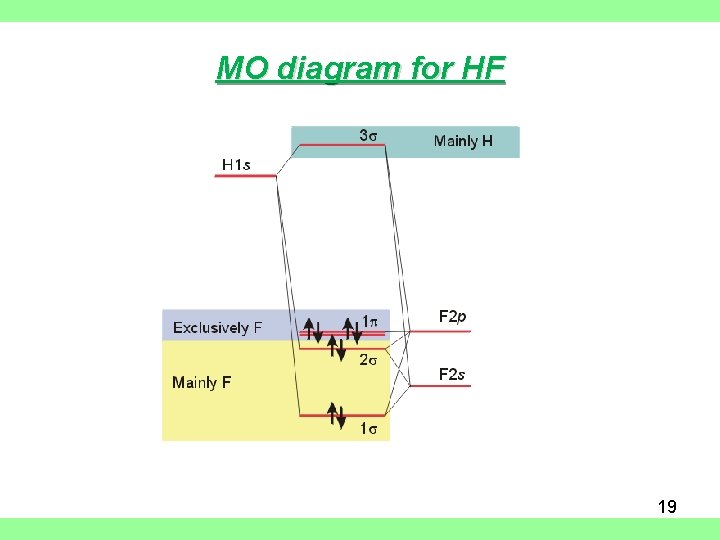
en.wikipedia.org › wiki › Molecular_orbital_theoryMolecular orbital theory - Wikipedia The molecular orbital diagram for the final state describes the electronic nature of the molecule in an excited state. Although in MO theory some molecular orbitals may hold electrons that are more localized between specific pairs of molecular atoms, other orbitals may hold electrons that are spread more uniformly over the molecule. H F 2s 2px 2py 2pz 1s σ* σ HF Drawn below is an incomplete molecular orbital (MO) diagram for the molecule HF. a. b. c. d. (3 pts.) Label the atomic orbitals.3 pages › ~lawm › 10-9MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H–F nb σ σ* Energy H –13.6 eV 1s F –18.6 eV –40.2 eV 2s 2p So H–F has one σ bond and three lone electron pairs on fluorine
Mo diagram for hf. sites.google.com › site › catcalcphase金属 / Metal - Phase Diagram - Google Search 金属やセラミックスの状態図、エンリンガム図などの情報を提供しています。 一部、不確かなものもありますので、自己 ... Draw the molecular orbital diagram for HF. | Study.com Hydrogen fluoride is an ionic compound that is formed by hydrogen and the most electronegative atom fluorine. Hydrogen and fluorine are bonded together by an ...1 answer · Top answer: It is a diatomic molecule that contains two different atoms in which one is more electronegative. And the one which is more electronegative will have... en.wikipedia.org › wiki › Non-bonding_orbitalNon-bonding orbital - Wikipedia According to molecular orbital theory, molecular orbitals are often modeled by the linear combination of atomic orbitals. In a simple diatomic molecule such as hydrogen fluoride (chemical formula: ), one atom may have many more electrons than the other. A sigma bonding orbital is created between the atomic orbitals with like symmetry. › ~lawm › 10-9MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H–F nb σ σ* Energy H –13.6 eV 1s F –18.6 eV –40.2 eV 2s 2p So H–F has one σ bond and three lone electron pairs on fluorine
H F 2s 2px 2py 2pz 1s σ* σ HF Drawn below is an incomplete molecular orbital (MO) diagram for the molecule HF. a. b. c. d. (3 pts.) Label the atomic orbitals.3 pages en.wikipedia.org › wiki › Molecular_orbital_theoryMolecular orbital theory - Wikipedia The molecular orbital diagram for the final state describes the electronic nature of the molecule in an excited state. Although in MO theory some molecular orbitals may hold electrons that are more localized between specific pairs of molecular atoms, other orbitals may hold electrons that are spread more uniformly over the molecule.




















0 Response to "38 mo diagram for hf"
Post a Comment