42 use the energy diagram for the reaction to answer the questions.
potential energy diagram Answer the questions using the graph above. 1. Is the above reaction endothermic or exothermic? exothermic. 2. What letter represents the potential energy of ...8 pages and mark scheme Class: - A. Hammond Biology The energy level diagram for this reaction is shown in Figure 3 . Figure 3 Mark clearly with a cross (x) on Figure 3 where bond breaking happens. (1) (Total 4 marks) Page 3 of 42. Kelp is a seaweed. Kelp can be burned to give out energy. € ©€Ethan Daniels/Shutterstock (a) €€€€Draw a ring around the correct answer to complete each sentence. € € endothermic. € Reactions …
Potential Energy Diagrams Worksheet Answers.pdf - SCH 4U ... your answer. Exothermic, the forward was endothermic ___ 2. Use the diagram below to answer the questions. a. Label reactants, products, heat of reaction (ΔH), activation energy for the forward reaction, activation energy for the reverse reaction and activated complex.
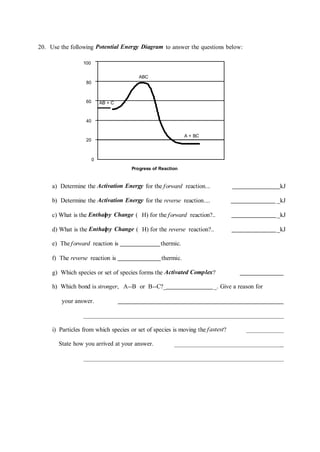
Use the energy diagram for the reaction to answer the questions.
Use the energy diagram for the reaction a d to answer the ... O exothermic O endothermic O cannot determine from the diagram Consider the mechanism. Step 1: A = B + C Step 2: C + D E Overall: A + D - B + E equilibrium slow Determine the rate law for the overall reaction, where the overall rate constant is represented as k. rate = A certain reaction has an activation energy of 56.02 kJ / mol. Reaction profiles - Exothermic and endothermic reactions ... An energy level diagram. shows whether a reaction is exothermic. or endothermic. It shows the energy in the reactants and products , and the difference in … Adenosine Triphosphate - Georgia State University Free Energy from Hydrolysis of ATP Adenosine triphosphate (ATP) is the energy currency of life and it provides that energy for most biological processes by being converted to ADP (adenosine diphosphate). Since the basic reaction involves a water molecule, ATP + H 2 O → ADP + P i. this reaction is commonly referred to as the hydrolysis of ATP.The change in Gibbs …
Use the energy diagram for the reaction to answer the questions.. Energy Diagram Practice - ScienceGeek.net Use this energy diagram to answer these questions. 1. The heat content of the reactants of the forward reaction is about kilojoules. 2. The heat content of the products of the forward reaction is about kilojoules. 3. The heat content of the activated complex of the forward reaction is about kilojoules. 4. Potential Energy Diagrams Question, Answer. Does the graph represent an endothermic or exothermic reaction? Endothermic. Determine the heat of reaction, DH, for this reaction. +50kJ.Mar 24, 2007 · Uploaded by kentchemistry.com [Solved] 3. Use the following reaction energy diagram to ... 3. Use the following reaction energy diagram to answer the questions: 70 60 A 150 4008 John Bettefeuil 40 B 30 Energy (kcal) Energy (kcal) 20 10 O Progress of reaction Progress of reaction a. Which curve represents the faster reaction, and which represents the slower? b. Use the reaction energy diagram above to a... | Clutch Prep Problem: Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, ΔG for the step C to B _____ kcal/mol Calculate the overall energy change, ΔG°, for the process B to A. _____kcal/mol.
PDF Potential Energy Diagrams Worksheet Potential Energy Diagrams Worksheet CK-12 Foundation Chemistry Name Use the following Potential Energy Diagram to answer questions 1 - 12. 150 X2Y2 100 Potential Energy (kJ) 50 0 Progress of Reaction 1. Is the overall reaction as shown exothermic or endothermic? 2. What is the activation energy for the forward reaction? 3. What is the ... study.com › learn › potential-energy-questions-andPotential Energy Questions and Answers | Study.com Draw an energy diagram that includes a second intermediate that is less stable than the first intermediate and shows that the third step of the reaction is faster than the reverse of the of the fir... Solved Use the energy diagram for the reaction A - D to ... We review their content and use your feedback to keep the quality high. Ans :- Given reaction is : A ----------> D Complete energy diagram of the reaction will be : Therefore, a). Number of transition …. View the full answer. Transcribed image text: Use the energy diagram for the reaction A - D to answer the questions. Answered: Use the reaction energy diagram above… | bartleby Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, ΔG, for the step A to B. kcal/mol. Calculate the overall energy change, ΔG°, for the process B to C. kcal/mol. Which step is faster, (a) A to B or (b) C to B. Transcribed Image Text: 25 20 15 10 D Reaction progress Free Energy (kcal/mol) LO.
DOC Chemistry 12 - Weebly USE THE POTENTIAL ENERGY DIAGRAM TO ANSWER THE QUESTIONS BELOW: 1. Is the overall reaction as shown . exothermic . or . endothermic ... the Activation Energy for the forward reaction and the Activation Energy for the reverse reaction on the graph above. 16. As reactant particles approach each other before a collision, the . Potential. Answered: Use the energy diagram for the reaction… | bartleby Use the energy diagram for the reaction A D to answer the questions. How many transition states are there in the reaction? How many intermediates are there in the reaction? Which step of the reaction is the fastest? Which step of the reaction has the smallest rate constant? Assume the frequency factor (?) is the same for each elementary reaction. PDF Potential Energy Diagram Worksheet ANSWERS Reaction Rates and Potential Energy Diagrams 1. Chemical reactions occur when reactants collide. For what reasons may a collision fail to produce a chemical reaction? Not enough energy; improper angle. 2. If every collision between reactants leads to a reaction, what determines the rate at which the reaction occurs? The diagram represents a spontaneous reaction. Use the ... 1. Cellular respiration is an exothermic reaction. Using appropriate terms to explain why this is important. 2 pt 2. Use the following diagram to answer the following questions. 1 point each EXAMINING THE DIAGRAM Ea Enthalpy Starting Energy AH Ending...
The diagram represents a spontaneous react... | Clutch Prep The diagram represents a spontaneous reaction. Use the diagram to answer the questions below. Is the reaction endothermic or exothermic? What is the activation energy of the reaction?
Potential Energy Diagrams Quiz - Quizizz Question 15. SURVEY. 30 seconds. Q. How much energy is released when two moles of methane (CH 4) reacts at 298 K and 101.3 kPa according to the following equation: CH 4 (g) + 2O 2 (g) → CO2(g) + 2H2O (l) answer choices. 890.4 J. 890.4 kJ.
Internal Energy Questions and Answers | Study.com Internal Energy Questions and Answers. Get help with your Internal energy homework. Access the answers to hundreds of Internal energy questions that are explained in a …
Answer: The diagram represents a spontaneo... | Clutch Prep Use the diagram to answer the questions below.a. Is the reaction endothermic or exothermic?b. What is the activation energy of the reaction? FREE Expert Solution Show answer Answer: a) exothermic; b) 25 kJ. 99% (496 ratings) Sign up for free to keep watching this solution Sign up for free ...
Answered: 25 20 15 10 Reaction progress Use the… | bartleby Science Chemistry Q&A Library 25 20 15 10 Reaction progress Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, AG, for the step B to C. kcal/mol Calculate the overall energy change, AG°, for the process A to B. kcal/mol Which step is faster, (a) C to B or (b) B to A? Free Energy (kcal/mol) B.
Solved Use the energy diagram for the reaction A D to ... Use the energy diagram for the reaction A D to answer the questions. How many transition states are there in the reaction? transition states: Potential energy How many intermediates are there in the reaction? intermediates: Reaction progress Which step of the reaction is the fastest? Which step of the reaction has the smallest rate constant?
Important Questions for Class 12 Chemistry Chapter 4 ... 06/12/2019 · The activation energy of the reaction decreases by the use of catalyst. Question 4. Express the rate of the following reaction in terms of the formation of ammonia : N 2 (g) + 3H 2 (g) → 2NH 3 (g) (Comptt. All India 2013) Answer: Question 5. If the rate constant of a reaction is k = 3 × 10-4 s-1, then identify the order of the reaction. (Comptt. All India 2013) Answer: S-1 is …
Free Body Diagram Questions and Answers - Study.com Free Body Diagram Questions and Answers. Get help with your Free body diagram homework. Access the answers to hundreds of Free body diagram questions that are explained in a way that's easy for ...
Class 11 Important Questions for Chemistry - Thermodynamics 22/04/2019 · Chemistry Important Questions Class 11 are given below. Multiple Choice Questions (Type-I) Thermodynamics is not concerned about_____. (i) energy changes involved in a chemical reaction. (ii) the extent to which a chemical reaction proceeds. (iii) the rate at which a reaction proceeds. (iv) the feasibility of a chemical reaction.
PDF Livingston Public Schools / LPS Homepage Use the energy diagram for the rearrangement reaction of methyl isonitrile to acetoni- trile to answer the following questions. 7. What kind of reaction is represented by this diagram, endothermic or exothermic? 8. What is the chemical structure identified at the top of the curve on the diagram? Cor«plc¥ 9. What does the symbol E represent? 10.
3. Use the potential energy diagram to help you answer the ... Use the potential energy diagram to help you answer the questions below. Make sure to show ALL work for calculations and provide an explanation when directed for full credit. What is the activation energy of this reaction? Show your work ! b. What is the total change in enthalpy of this reaction? Show your work! . Is the slope positive or negative?
Chemistry Theory Use the following energy profile diagram ... Use the following energy profile diagram to answer Questions (a) to (c) below. (a) From the diagram, determine the value of the; (i) enthalpy of the reaction (ii) activation energy of the reaction. (b) State whether the profile diagram is for an endothermic reaction or an exothermic reaction.
Answered: Use the energy diagram for the reaction… | bartleby Solution for Use the energy diagram for the reaction A → D to answer the questions. How many transition states are there in the reaction? transition ...1 answer · Top answer: Step 1 Since you have posted a question with multiple sub-parts, we will solve first three subparts for you. To get the remaining sub-parts solved, ...
Application and Practice Questions - Physics Classroom Use your understanding of the work-energy theorem to answer the following questions. Then click the button to view the answers. 1. Consider the falling and rolling motion of the ball in the following two resistance-free situations. In one situation, the ball falls off the top of the platform to the floor. In the other situation, the ball rolls from the top of the platform along the staircase ...
PDF Chemical Kinetics - GUSD Examine the following Potential Energy Diagrams then answer the questions that follow. w + v y 1. Which of the reactions has the greatest H (or heat of reaction)? _____ 2. Which reaction has the greatest Activation Energy? _____ 3. For which reactions might we want to use a catalyst to increase the rate of reaction? _____ 4.
Chemistry Unit 6 Flashcards - Quizlet Use the potential energy diagram below to answer the following question The potential energy of the reactants is ____kJ, while the potential energy of the products is _____. ( straight, wayyyyyyyyyyy uphill, then tiny downhill, then straight )
Energy Diagram Practice - ScienceGeek.net Energy Diagram Practice Gap-fill exercise Fill in all the gaps, then press "Check" to check your answers. Use this energy diagram to answer these questions. 1. The enthalpy of the reactants of the reaction is about kilojoules. 2. The enthalpy of the products of the reaction is about kilojoules. 3.
Drawing Free-Body Diagrams - Physics Classroom Diagram the forces acting on the combination of gymnast and bar. A free-body diagram for this situation looks like this: Return to Questions. Return to Info on Free-body diagrams. Return to on-line Force Description List . 3. An egg is free-falling from a nest in a tree. Neglect air resistance. A free-body diagram for this situation looks like ...
PDF Chemistry*12* Name:* Potential*Energy*Diagrams*Worksheet Chemistry*12* Potential*Energy*Diagrams*Worksheet* Name:* Date:* Block:*! USE!THE!POTENTIAL*ENERGY*DIAGRAM!TO!ANSWER!THEQUESTIONS!BELOW:! 1.! Is!the!overall!reaction ...
K1 Packet pages 15 to 25.pdf Use this reaction to answer questions 18 & 19: H2(g) 1/2O2(g) ... Base your answers to questions 24 - 26 on the potential energy diagram shown below:.12 pages
Adenosine Triphosphate - Georgia State University Free Energy from Hydrolysis of ATP Adenosine triphosphate (ATP) is the energy currency of life and it provides that energy for most biological processes by being converted to ADP (adenosine diphosphate). Since the basic reaction involves a water molecule, ATP + H 2 O → ADP + P i. this reaction is commonly referred to as the hydrolysis of ATP.The change in Gibbs …
Reaction profiles - Exothermic and endothermic reactions ... An energy level diagram. shows whether a reaction is exothermic. or endothermic. It shows the energy in the reactants and products , and the difference in …
Use the energy diagram for the reaction a d to answer the ... O exothermic O endothermic O cannot determine from the diagram Consider the mechanism. Step 1: A = B + C Step 2: C + D E Overall: A + D - B + E equilibrium slow Determine the rate law for the overall reaction, where the overall rate constant is represented as k. rate = A certain reaction has an activation energy of 56.02 kJ / mol.
0 Response to "42 use the energy diagram for the reaction to answer the questions."
Post a Comment