42 ni2+ orbital diagram
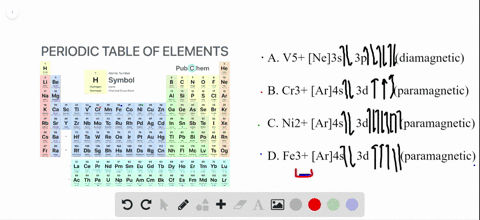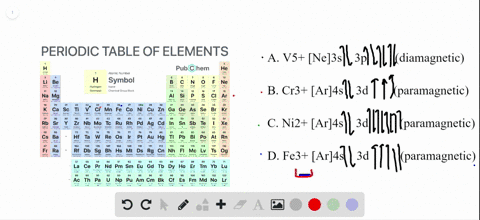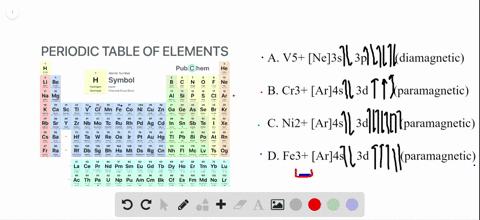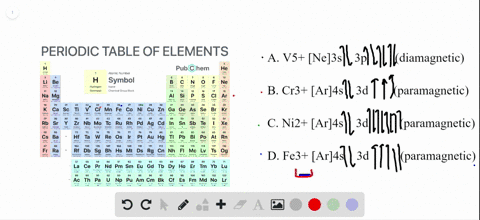
SOLVED:Write orbital diagrams for each ion and determine ... Write orbital diagrams for each ion and determine if the ion is diamagnetic or paramagnetic. a. V5+ b. Cr3+ c. Ni2+ d. Fe3+ Write orbital diagrams for each ion and indicate whether ... Explanation. Verified. Step 1. 1 of 6. In writing orbital diagrams, first, determine the electron configuration of the neutral atom and remove electrons accordingly. Diamagnetic ions contain no unpaired electrons. Paramagnetic ions have unpaired electrons. Determine the orbital diagrams of a. V.
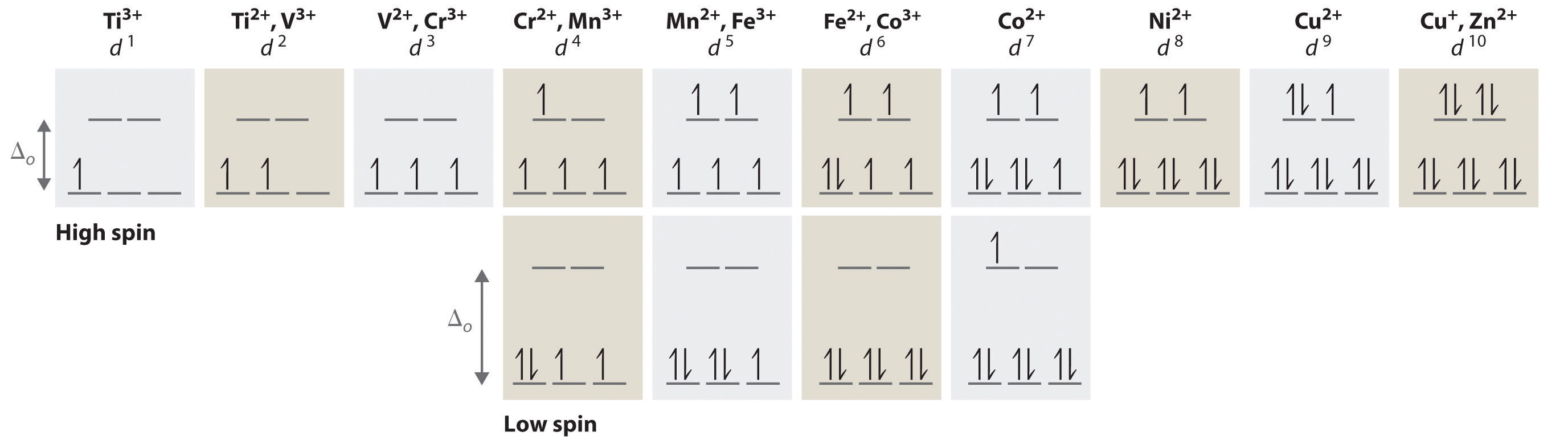
Draw The Orbital Diagram For The Ion Co2+ - schematron.org Ni2+ Draw the d-orbital splitting diagrams for the octahedral complex ions of each of the following. a. Zn2+ b. Co2+ (high and low spin) c. Ti3+ the FT ligand. Why isn't it [Ar] 4s2 3d5 (considering the fact that half filled orbitals confer If you have a gas phase Co (2+) ion, it would likely reorganize such.

Ni2+ orbital diagram
OneClass: For Ni2+, draw an orbital energy diagram and ... For Ni2+, draw an orbital energy diagram and place the valence electrons in the diagram that would indicate that it is in an excited state. Transition Metal Chemistry and Paper Chromatography would indicate that it is an excited state, Show full question Answer + 20 Watch For unlimited access to Homework Help, a Homework+ subscription is required. Orbital Diagram For V5+ - schematron.org Answer to Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+ Determine if the ion is diamagnetic or paramagnetic. V 86%(14). Write orbital diagrams for each of these ions. A. V^5+ B. Cr^3+ C. Ni^2+ D. Fe^3+ E. Determine if the following ions are diamagnetic or paramagnetic. QUESTIONS BASED ON HIGH ORDER THINKING SKILL It undergoes d² sp³ hybridisation. Each hybrid orbital receives 1 pair of electrons from ammonia. Since all electrons are paired it is diamagnetic. In [CoF6]3+, cobalt ion is in + 3 oxidation state and has the electronic configuration 3d6. It undergoes sp³ d² hybridisation. Each hybrid orbital receives a pair of electrons from F–.
Ni2+ orbital diagram. Energy level diagram for Ni2+. why the electrons are taken ... Energy level diagram for Ni2+. why the electrons are taken from s orbital but not d orbital? Chemistry. Answer Comment. ... than the mentioned d-orbital (n=3). That means the s-orbital will loose the electrons first (the valence electrons are in n=4, so they will be the first to go way) Send. Solved 1. Write the electronic configuration of Ni2+. 2 ... 1. Write the electronic configuration of Ni2+. 2. Draw an orbital energy level diagram for the electrons in Ni2+. Question: 1. Write the electronic configuration of Ni2+. 2. Draw an orbital energy level diagram for the electrons in Ni2+. PDF Orgel and Tanabe-Sugano Diagrams for Transition Metal ... Orgel Diagrams Orgel diagrams are the oversimplified version of correlation diagrams that show the relative energies of electronic terms in transition metal complexes. They are named after their inventor, Leslie Orgel. These diagrams are restricted only to show weak field cases and offer no information about strong field cases. (PDF) Inorganic Chemistry by Miessler ~ 5th Edition ... This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry. (PDF) Inorganic Chemistry by Miessler ~ 5th Edition | Arnab Patra - Academia.edu
Orbital filling diagrams - The Cavalcade o' Chemistry The orbital filling diagram of boron. I skipped past beryllium because I was getting bored. The electron configuration of boron is 1s²2s²2p¹, which means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbitals. This gives us an orbital filling diagram of: [email protected] - casinospieleslots.de Mar 09, 2022 · email protected] [email protected] Solved 2. For Cr and Ni2+ a. Write the electron | Chegg.com 2. For Cr and Ni2+ a. Write the electron configuration. b. Write the orbital diagram for the valence electrons. c. How many unpaired electrons does each have? Question: 2. For Cr and Ni2+ a. Write the electron configuration. b. Write the orbital diagram for the valence electrons. c. How many unpaired electrons does each have? Giant orbital polarization of Ni2+ in a square planar ... @article{osti_1787634, title = {Giant orbital polarization of Ni2+ in a square planar environment}, author = {Mandal, Prithwijit and Patel, Ranjan Kumar and Rout, Dibyata and Banerjee, Rajdeep and Bag, Rabindranath and Karmakar, Koushik and Narayan, Awadhesh and Freeland, John W. and Singh, Surjeet and Middey, Srimanta}, abstractNote = {Finding large orbital polarization in Ni-based oxides has ...
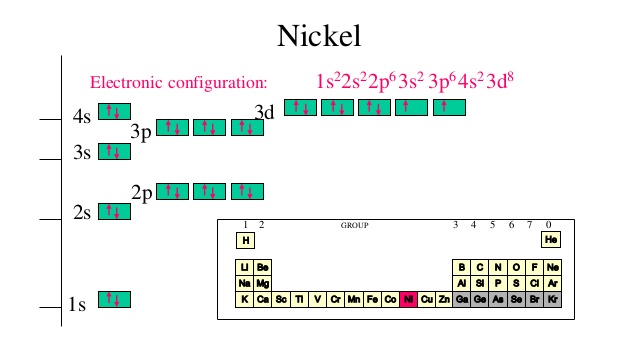
What is the electron configuration of Ni^(2+)? | Socratic Explanation: Electronic configuration of Nickel ( 28Ni) is. 1s2 2s2 2p6 3s2 3p6 4s2 3d8. After removal of two electrons from outermost shell the electron configuration of Ni2+ is. 1s2 2s2 2p6 3s2 3p6 4s0 3d8. Answer link. PDF Orbital Diagrams, Noble Gas Configuration, Lewis Dot Diagrams Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom What is the orbital diagram for nickel? - Quora Answer (1 of 4): Nickel is atomic number 28; therefore, it has 28 electrons in its orbitals. The filling rules are as follows: 1. Aufbau Principle: Lowest energy levels fill first. 2. Pauli Exclusion Principle: Only 2 electrons per orbital, they must have opposite spin. 3. Hund's Rule: Given sev... MO Diagram for N2+ (Molecular Orbital) - YouTube There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc).One is for the elements up to Nitrogen. The other is for AFTER nitrogen (start...
DOC CH186 - Southeast Missouri State University 5) For the following complex, draw an orbital diagram for the isolated metal ion. Then, using valence bond theory, draw the orbital diagram for the metal ion in the complex and indicate the geometry of the complex. Also, clearly indicate which hybrid orbitals the metal uses for covalent bonding with the ligands. [FeCl4]- (5 unpaired electron)
BITSAT 2022 Exam - Dates, Admission Process, Eligibility ... Mar 08, 2022 · Covalent bond: Valence bond theory-orbital overlap, directionality of bonds and hybridization (s, p, and d orbitals only), resonance, molecular orbital theory-methodology, orbital energy level diagram, bond order: Covalent bond: Magnetic properties for homonuclear diatomic species (qualitative idea only) Dipole moments, hydrogen bond
Electron Configuration for Chromium (Cr, Cr2+, Cr3+) After the 4s is full we put the remaining four electrons in the 3d orbital and end with 3d4. Therefore the expected electron configuration for Chromium will be 1s 2 2s 2 2p 6 3s 2 3p 4 4s 2 3d 9. Note that when writing the electron configuration for an atom like Cr, the 3d is usually written before the 4s.
Nickel atom can lose two electrons to form Ni^2 + ion. The ... To form N i 2 +, it will lose two electrons from 4s orbital to form N i 2 + with electronic configuration [A r] 1 8 3 d 8 4 s 0. Solve any question of Structure of Atom with:- Patterns of problems
SOLVED:Write orbital diagrams for each ion and determine ... take a look here at utilizing the periodic table too. Do some orbital configurations or diagrams for some metal cat ions. So first example is going to be for chromium three plus. So as we follow the periodic table and fill the electrons properly, first row of the periodic table. Um we feel the first two electrons in the structure in the one S sub shell with its one orbital.
electronic configuration - Molecular orbital (MO) diagram ... Molecular orbital diagram for nitrogen monoxide, the nitrosyl cation and the nitrosyl anion. 6. What is the correct molecular orbital diagram for the d orbitals in platinum for the tetraammineplatinum(II) complex? Hot Network Questions Why can't Russia stop Ukraine from joining NATO by signing a treaty with a friendly NATO state?
(PDF) Complete Solutions Manual GENERAL ... - Academia.edu Academia.edu is a platform for academics to share research papers.
ACS Omega | Vol 7, No 2 Jan 18, 2022 · The motion of a <0001>{1–100} screw dislocation in a single crystal of AlN is explored by molecular dynamics simulations using LAMMPS. Four modes of thermally activated motion are observed under different conditions of temperature and stress: double kinks, Shockley partials, self-pinning, and debris and dislocation loops. View the article.
How many unpaired electrons are present in Ni2 ... - Quora The electronic configuration of nitrogen atom is 1s2 2s2 2p3. The molecular orbital diagram is: From the electronic configuration of Nitrogen molecule, we can say that there is no unpaired electron in the nitrogen molecule. Since the bond order is 3, it means that there is a triple bond between two nitrogen atom in the molecule.
Ni2+ Electron Configuration - Student Doctor Network Ni2+ Electron Configuration | Student Doctor Network. Advisor of the Year Nominations. Show your pre-health or career advisor some love - nominate them for the HPSA Advisor of the Year competition! HPSA Student Advisory Council will consider exceptional advisors for recognition. Nominations now thru 3/18.
Inorganic Chemistry | Vol 61, No 9 Mar 07, 2022 · Actinyl ions (AnO22+), with their linear geometry, chemical stability, and prevalence in a wide range of solid and solution state forms, exemplify the distinctive electronic structure of the actinide elements. Spectroscopic measurements of field gradient tensors and magnetic parameters in crystalline samples of Cs2UO2Br4 (left) and Cs2UO2Cl4 (right) in conjunction with ab initio calculations ...
Nickel(Ni) electron configuration and orbital diagram Atomic Orbital Diagram for Nickel (Ni) Nickel(Ni) excited state electron configuration. Atoms can jump from one orbital to another orbital by excited state. This is called quantum jump. Ground state electron configuration of nickel is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 8 4s 2.
Schematic level diagrams of the Ir4+ 5d and Ni2+ 3d ... Schematic level diagrams of the Ir4+ 5d and Ni2+ 3d orbitals. The down-spin 3z2 − r2 electron mediates a FM coupling via a ddσ hybridization [ (a) and (b)]. The up-spin xz/yz electrons mediate an...
Flour Mill Rye [4MH368] Search: Rye Flour Mill. What is Rye Flour Mill. Every flour has its own unique properties. Sourdough Rye using your flour and some crushed organic caraway seeds has lifted my Sourdough Rye to a new level!!
Lewis acids and bases - Wikipedia Classically, the term "Lewis acid" is restricted to trigonal planar species with an empty p orbital, such as BR 3 where R can be an organic substituent or a halide. [citation needed] For the purposes of discussion, even complex compounds such as Et 3 Al 2 Cl 3 and AlCl 3 are treated as trigonal planar Lewis acids.Metal ions such as Na +, Mg 2+, and Ce 3+, which are invariably …
Tetrahedral complexes energy level diagram - Big Chemical ... Tetrahedral complexes energy level diagram In a nickel-containing enzyme various groups of atoms in the enzyme form a complex with the metal, which was found to be in the +2 oxidation state and to have no unpaired electrons.What is the most probable geometry of the Ni2+ complex (a) octahedral (b) tetrahedral (c) square planar (see Exercise 16.96) Justify your answer by drawing the orbital ...
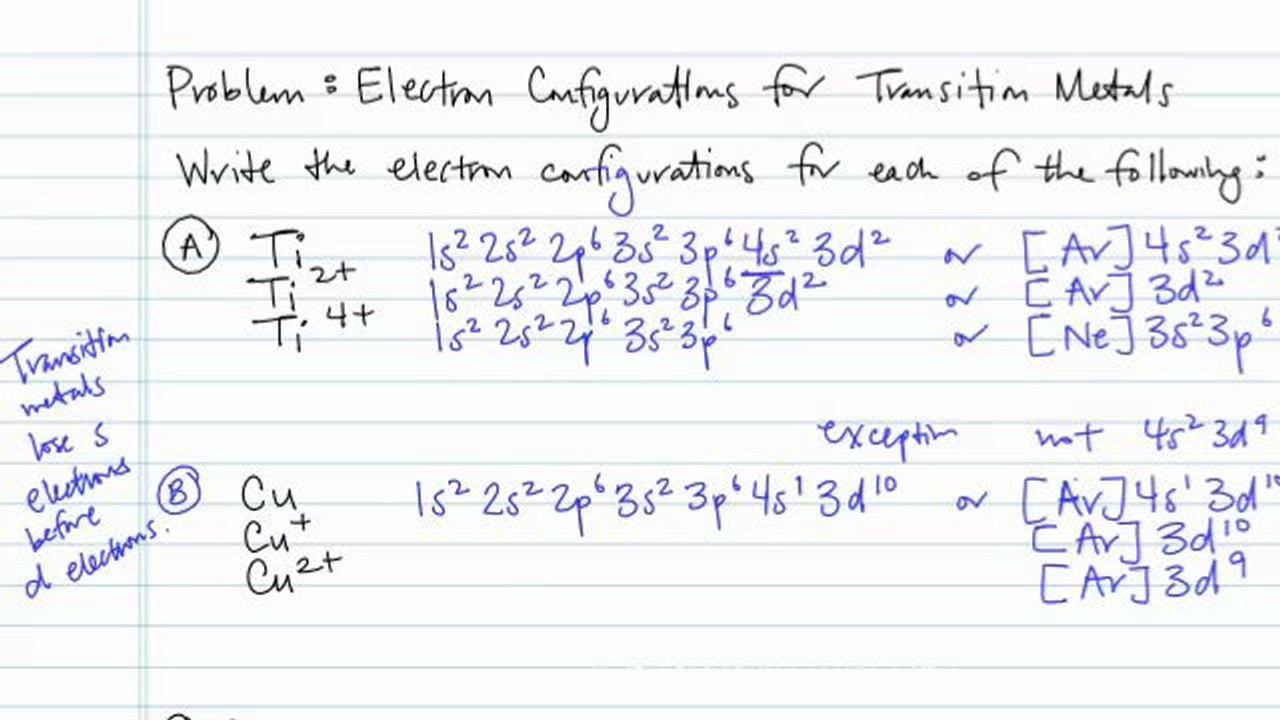
Neon(Ne) electron configuration and orbital diagram To write the orbital diagram of neon(Ne), you have to do the electron configuration of neon. Which has been discussed in detail above. Neon (Ne) orbital diagram. 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in ...
Energy Transfer in Metal–Organic Frameworks for ... Feb 17, 2022 · The development of materials with outstanding performance for sensitive and selective detection of multiple analytes is essential for the development of human health and society. Luminescent metal–organic frameworks (LMOFs) have controllable surface and pore sizes and excellent optical properties. Therefore, a variety of LMOF-based sensors with diverse detection functions can be easily ...
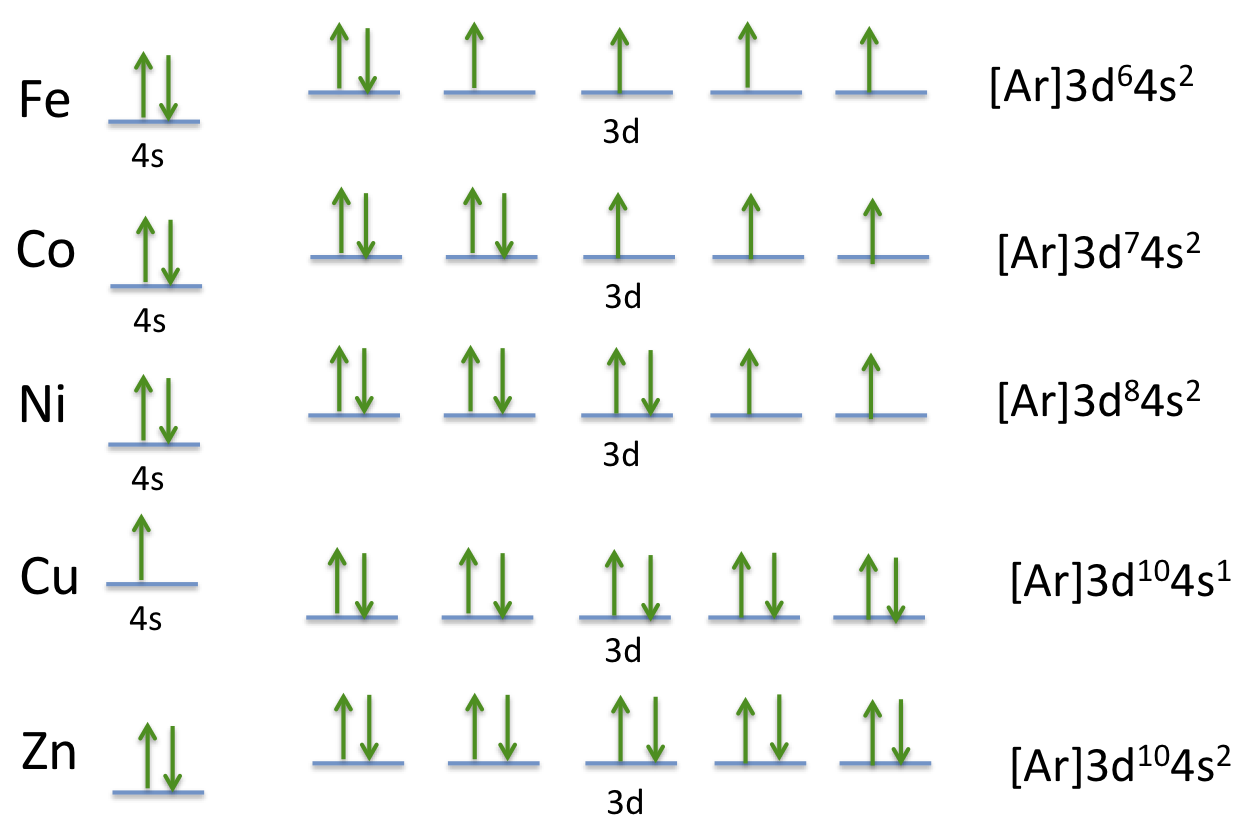
Electron Configuration for Ni, Ni2+, and Ni3+ (Nickel and ... To write the configuration for the Nickel ions, first we need to write the electron configuration for just Nickel (Ni). We first need to find the number of ...
ni2+ paramagnetic or diamagnetic Write orbital diagrams for each ion and indicate whether the ion is diamagnetic or paramagnetic. Now, Ni +2 ion is 3d8 system. Interpretation: The paramagnetic or diamagnetic behaviour of the given metal complexes [Fe (CN) 6] 4 − and [Fe (H 2 O) 6] 2 + is to be stated.
QUESTIONS BASED ON HIGH ORDER THINKING SKILL It undergoes d² sp³ hybridisation. Each hybrid orbital receives 1 pair of electrons from ammonia. Since all electrons are paired it is diamagnetic. In [CoF6]3+, cobalt ion is in + 3 oxidation state and has the electronic configuration 3d6. It undergoes sp³ d² hybridisation. Each hybrid orbital receives a pair of electrons from F–.
Orbital Diagram For V5+ - schematron.org Answer to Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+ Determine if the ion is diamagnetic or paramagnetic. V 86%(14). Write orbital diagrams for each of these ions. A. V^5+ B. Cr^3+ C. Ni^2+ D. Fe^3+ E. Determine if the following ions are diamagnetic or paramagnetic.
OneClass: For Ni2+, draw an orbital energy diagram and ... For Ni2+, draw an orbital energy diagram and place the valence electrons in the diagram that would indicate that it is in an excited state. Transition Metal Chemistry and Paper Chromatography would indicate that it is an excited state, Show full question Answer + 20 Watch For unlimited access to Homework Help, a Homework+ subscription is required.




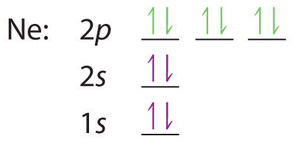

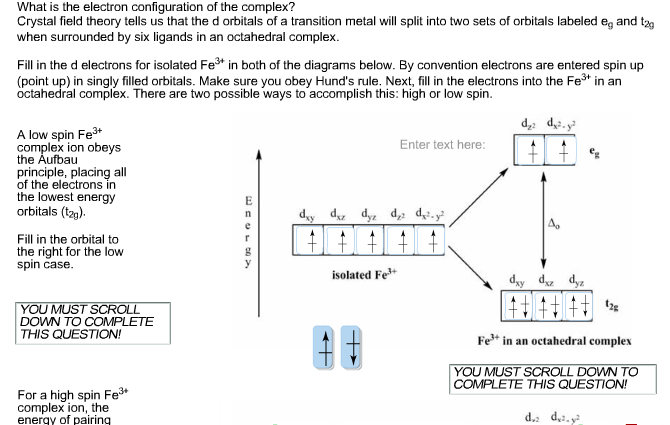







![The following orbital diagram describes: 3d 4s [Ar] [i ...](https://cdn.numerade.com/previews/4cc0eea8-2e8c-45d8-a4f7-03792b00c68d_large.jpg)




0 Response to "42 ni2+ orbital diagram"
Post a Comment