42 invariant point phase diagram
Invariant Point - an overview | ScienceDirect Topics The solid-liquid equilibrium phase diagram is generated according to the following steps 1-4. Step 1: System classification. Classify the system as single salt system or multiple salts from the data of the types of salts. Step 2: Invariant point calculation Invariant points for salt solutions - Phasediagram According to the phase rule, a one component system has no degrees of freedom when three phases are in equilibrium (F=0).The system is invariant. The triple point of water is at 273.16 K and 612 Pa. Phase diagram for water showing the triple point of water. This is an invariant point. This phase diagram is from Wikipedia. Binary systems
PDF Lecture 19: 11.23.05 Binary phase diagrams A region of the copper-zinc phase diagram that has been enlarged to show eutectoid and peritectic invariant points , C, 74 wt% Zn) and P (598 C, 78.6 wt% Zn), respectively. Figure by MIT OCW.
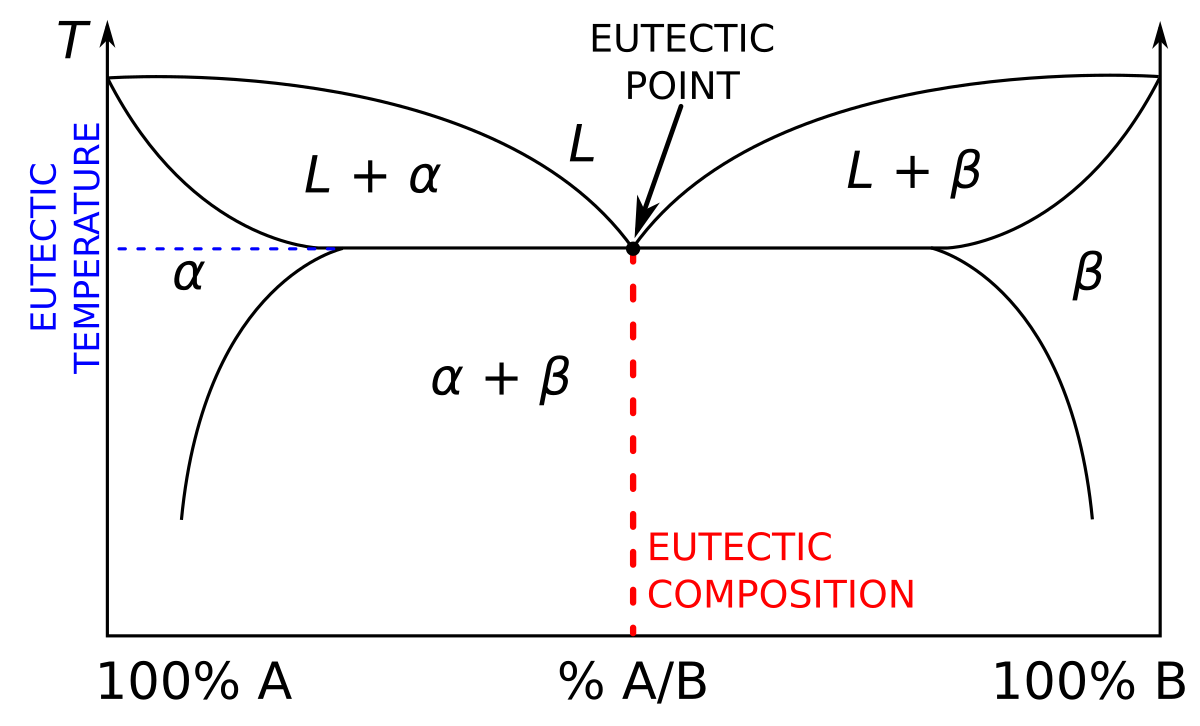
Invariant point phase diagram
PDF Ternary Phase Diagrams - University of South Alabama Phase Diagram Summary • Be familiar with all of the phase diagram assignments • Know the "modified" phase rule for igneous phase diagrams • Be able to explain why an invariant point on a phase diagram is a peritectic or eutectic. • Be able to explain the difference between a regular cotectic and reaction cotectic 2 Component Phase Diagrams - Tulane University invariant point. Peritectic point- The point on a phase diagram where a reaction takes place between a previously precipitated phase and the liquid to produce a new solid phase. When this point is reached, the temperature must remain constant until A peritectic is also an invariant point. Solved 12. The aluminum-nickel phase diagram has been ... The aluminum-nickel phase diagram has been provided. Identify all of the invariant points that exist in this system by the temperature and composition of the invariant point. If no example of a particular case exists, please state "none" or "not applicable." IGNORE the region around the invariant point at 86.7 wt% Ni at this time.
Invariant point phase diagram. Ternary Phase Diagrams - Tulane University Knowing what phases must be present in the subsolidus assemblage for any composition in the system is important, because it tells us where the crystallization path will lead, i.e. to which of the invariant points in the system where 3 solid phases and a liquid will coexist prior to the disappearance of the liquid phase. Schreinemakers Method - Teaching Phase Equilibria For a PT diagram, what the phase rule tells us is that: Invariant points occur at a fixed P and T. (You are NOT free to vary either if you wish to stay at the point.) Reaction lines occur over a range of P and T, but the two cannot be varied independently. Invariant eutectic point - Big Chemical Encyclopedia There are three invariant points in the iron-carbon diagram (Figures 4.16 and 4.17). A eutectic point is found at 4.30 wt% carbon and 1148 °C. At a eutectic point, a liquid transforms to two solids on cooling ... [Pg.104] A eutectic point on a binary phase diagram is (a) An invariant point... [Pg.108] What is invariant system in phase rule ... What is invariant point? Invariant Point: a point on a graph that remains unchanged after a transformation is applied to it. Any point on a line of reflection is an invariant point. What is isomorphous phase diagram? Phase diagrams represent the relationship between temperature and the composition of phases present at equilibrium.
Invariant Equilibrium - an overview | ScienceDirect Topics The LiF-NaF-KF phase diagram was measured by Bergmann and Dergunov, 45 who found the ternary eutectic with the lowest melting point at T = 727 K and LiF-NaF-KF (46.5-11.5-42.0 mol%). Thermodynamic assessment of this system was done in several studies, 46-48 all of which were in close agreement. 09mae324 - PD30 - Princeton University Phase Diagrams Invariant Reactions · The possible types of invariant reaction are shown below. The vertical green bar identifies the composition required for the invariant reaction, the horizontal line is the temperature of the reaction, and the phase points on the isotherm represent their compositions during the reaction. After: Askland, Phase Diagram: Meaning and Types | Material Engineering Point E on the phase diagram it is called invariant point at this point an important reaction takes place at constant temperature Called Eutectic reaction where liquid (L) is directly converted to (α) and β phase. iii. Line CEG is called eutectic isotherm. PDF Lecture30 Phase Diagrams - MIT OpenCourseWare MIT3.00Fall2002°c W.CCarter 204 Classifying the Invariant Points: Drawing Phase Diagrams There are two fundamental ways that invariant points can arise:29 1. When two two-phase regions join at a temperature and become one two-phase region: Eutectic (fi +liquid)+(liquid+ fl) *) (fi +fl)Eutectoid (fi +°)+(° +fl) *) (fi +fl)Figure 30-14: Eutectic-type (EV-TYPE at MASSACHVSETTS INSTITVTE OF
Eutectic Point - Soft-Matter - Harvard University Eutectic Point. Figure 1 shows a common and relatively simple binary phase diagram known as a eutectic phase diagram. A eutectic diagram can be thought of as the intersection of two solid solution diagrams. At the intersection of the two liquidus lines, the melt is in equilibrium with the two solid phases. In other words, a single liquid phase ... What is an invariant point in a phase diagram? - AnswersToAll What is an invariant point in a phase diagram? A eutectic is an invariant point. Peritectic point - The point on a phase diagram where a reaction takes place between a previously precipitated phase and the liquid to produce a new solid phase. When this point is reached, the temperature must remain constant until the reaction has run to completion. Phase diagrams for binary salt solutions : Phasediagram The two points B and C represent solutions in equilibrium with two solid phases and a gas phase and therefore constitute invariant points. Phase diagram for the CaCl 2 - H 2 O system. Experimental values marked with red circles are plotted together with the solubility line calculated with the Extended UNIQUAC model 250+ TOP MCQs on Phase Diagram and Answers Clarification: A unary phase diagram has a single component thus, at triple point, the number of degrees of freedom, according to Gibbs phase rule F = 1 - 3 + 2 = 0. 3. The invariant reaction involving, a liquid phase decomposing into two different solids on cooling is known as _________
invariant point | phase change | Britannica phase diagrams In phase: Unary systems Point C is therefore an invariant point; a change in either pressure or temperature results in the loss of one or more phases. The phase rule also reveals that no more than three phases can stably coexist in a one-component system because additional phases would lead to negative variance. Read More
Classifying the Invariant Points: Drawing Phase Diagrams The invariant points determine the topology of the phase diagram: Figure 30-16: Construct the rest of the Eutectic-type phase diagram by connecting the lines to the appropriate melting points. Figure 30-17: Construct the rest of Peritectic-type phase diagram, on the left a rule for all phase diagrams is illustrated--the ``lines'' must ...
Solved and peritectoid. How many degrees of ... - Chegg Transcribed image text: and peritectoid. How many degrees of freedom exist at invariant reaction points in binary phase diagrams? 2. Is Points) Write equations for the following invariant reactions: eutectic, eutectoid, peritectic, oints] Draw time-temperature cooling paths for a 1080 steel on an isothermal formation diagram that will produce the following microstructures.
Phase Rule - Teaching Phase Equilibria Invariant equilibria, in which neither P or T can be changed; on a phase diagram, this is represented as a singular invariant point Univariant equilibria, in which either P or T can be changed independently, but to maintain the state of the system, there must be a corresponding change in the other variable; on a phase diagram this is referred ...
PDF Ternary Phase Diagrams - Institute for Advanced Study Understanding Phase Diagrams - V.B. John . Ternary phase diagram books by D.R.F. West - there are several . Ternary grid . ... Grey plane is the invariant plane, where the invariant reaction occurs Ternary eutectic reaction . Space diagram and isothermal sections (a)
What is the peritectic point? - AskingLot.com Peritectic point - The point on a phase diagram where a reaction takes place between a previously precipitated phase and the liquid to produce a new solid phase. When this point is reached, the temperature must remain constant until the reaction has run to completion. A peritectic is also an invariant point.
PDF Geochemical Phase Diagrams and Gale Diagrams Phase diagram topologies for chemical systems with m n+2 are fairly well-understood, even as m grows large. For m n+1 they are essentially trivial, and for m = n+2, they look roughly like Figure 1, having an invariant point surrounded by several univariant reaction curves. The schematic picture of such an invariant point surrounded
PPTX PowerPoint Presentation Invariant Phase Equilibria. There are three degrees of freedom for a binary system. If three phases meet the conditions are invariant. This means that the phase composition acts like a pure component. An azeotrope or congruent melting point splits the phase diagram into two phase diagrams. There are several types of "invariant reactions ...
Lecture 5: Identifying Invariant Points on the Al-Ni Phase ... Al-Ni Phase Diagram analysis
PDF Phase Diagrams - University of Cincinnati An azeotrope or congruent melting point splits the phase diagram into two phase diagrams. There are several types of "invariant reactions". Azeotrope Congruent melting point Hex to Cubic Phase transition of Y2O3 PeritecticReaction Peritectoid = 3 solid phases Eutectic Reaction Eutectoid = liquid & 2 solid phases Monotectic Reaction L1=> S + L2
Solved 12. The aluminum-nickel phase diagram has been ... The aluminum-nickel phase diagram has been provided. Identify all of the invariant points that exist in this system by the temperature and composition of the invariant point. If no example of a particular case exists, please state "none" or "not applicable." IGNORE the region around the invariant point at 86.7 wt% Ni at this time.
2 Component Phase Diagrams - Tulane University invariant point. Peritectic point- The point on a phase diagram where a reaction takes place between a previously precipitated phase and the liquid to produce a new solid phase. When this point is reached, the temperature must remain constant until A peritectic is also an invariant point.
PDF Ternary Phase Diagrams - University of South Alabama Phase Diagram Summary • Be familiar with all of the phase diagram assignments • Know the "modified" phase rule for igneous phase diagrams • Be able to explain why an invariant point on a phase diagram is a peritectic or eutectic. • Be able to explain the difference between a regular cotectic and reaction cotectic

0 Response to "42 invariant point phase diagram"
Post a Comment