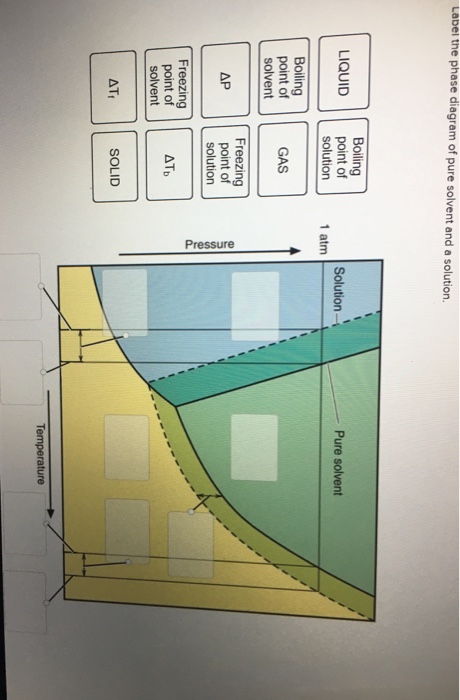
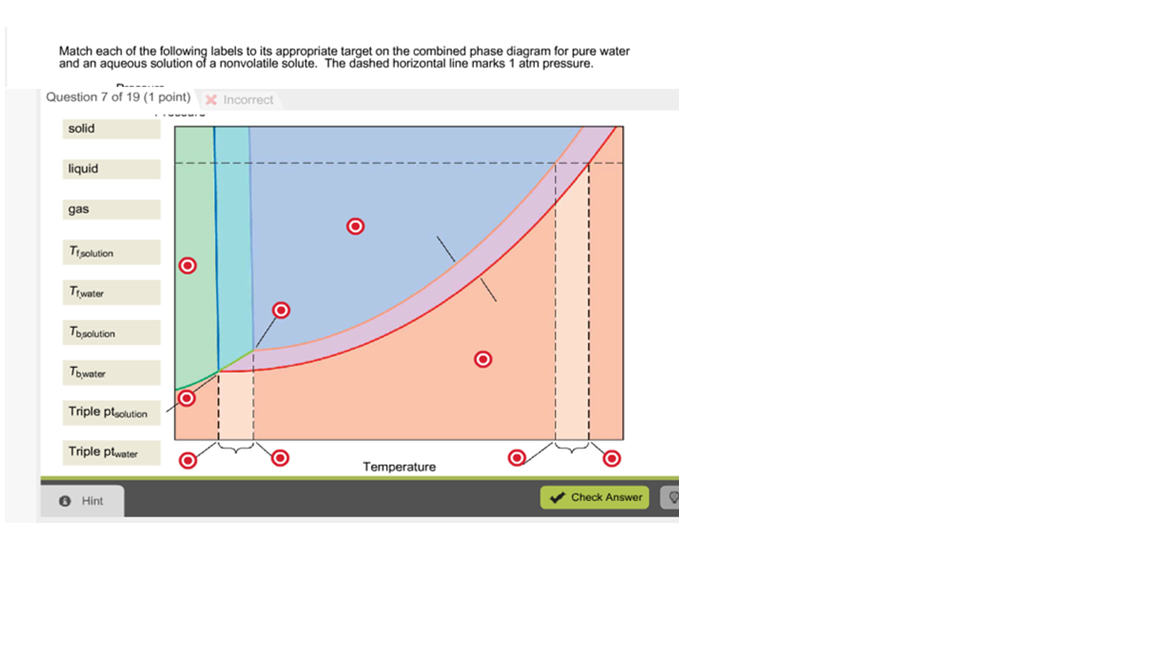
39 label the phase diagram of pure solvent and a solution.
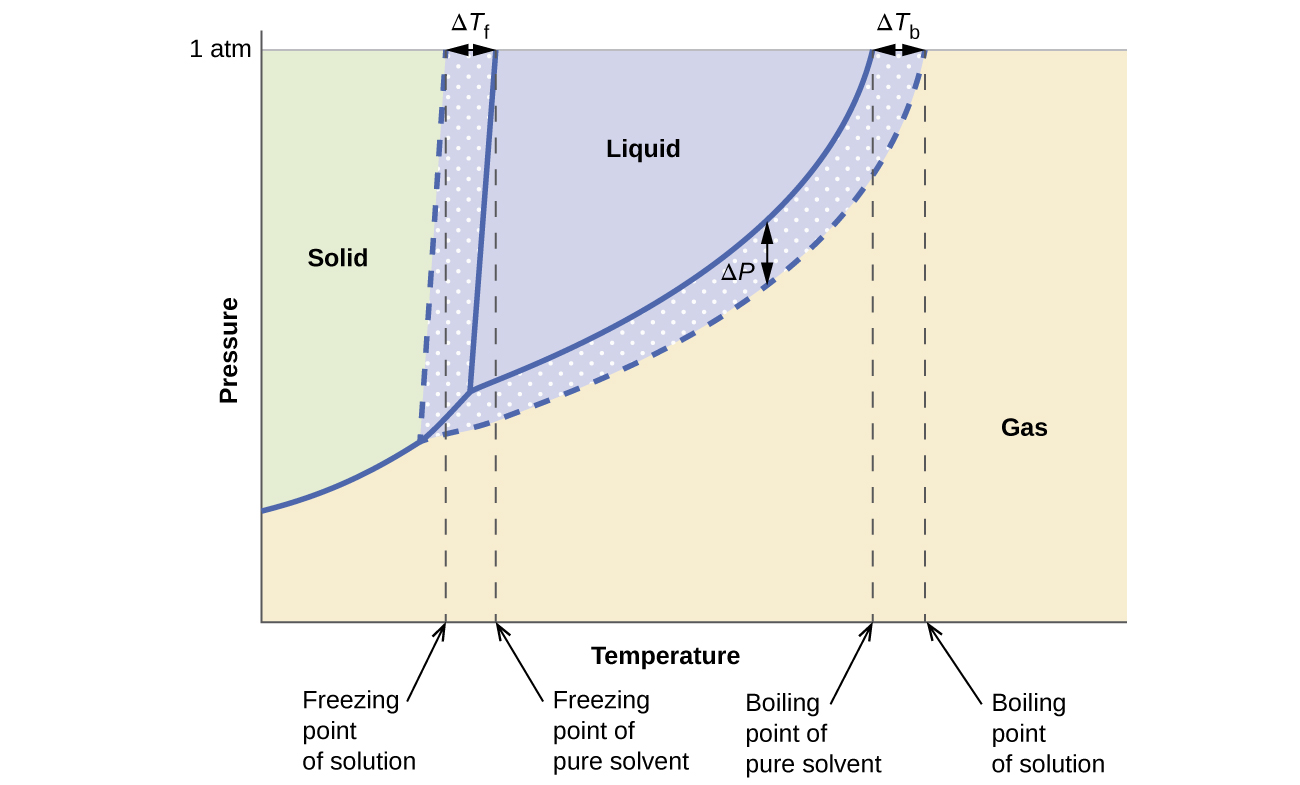
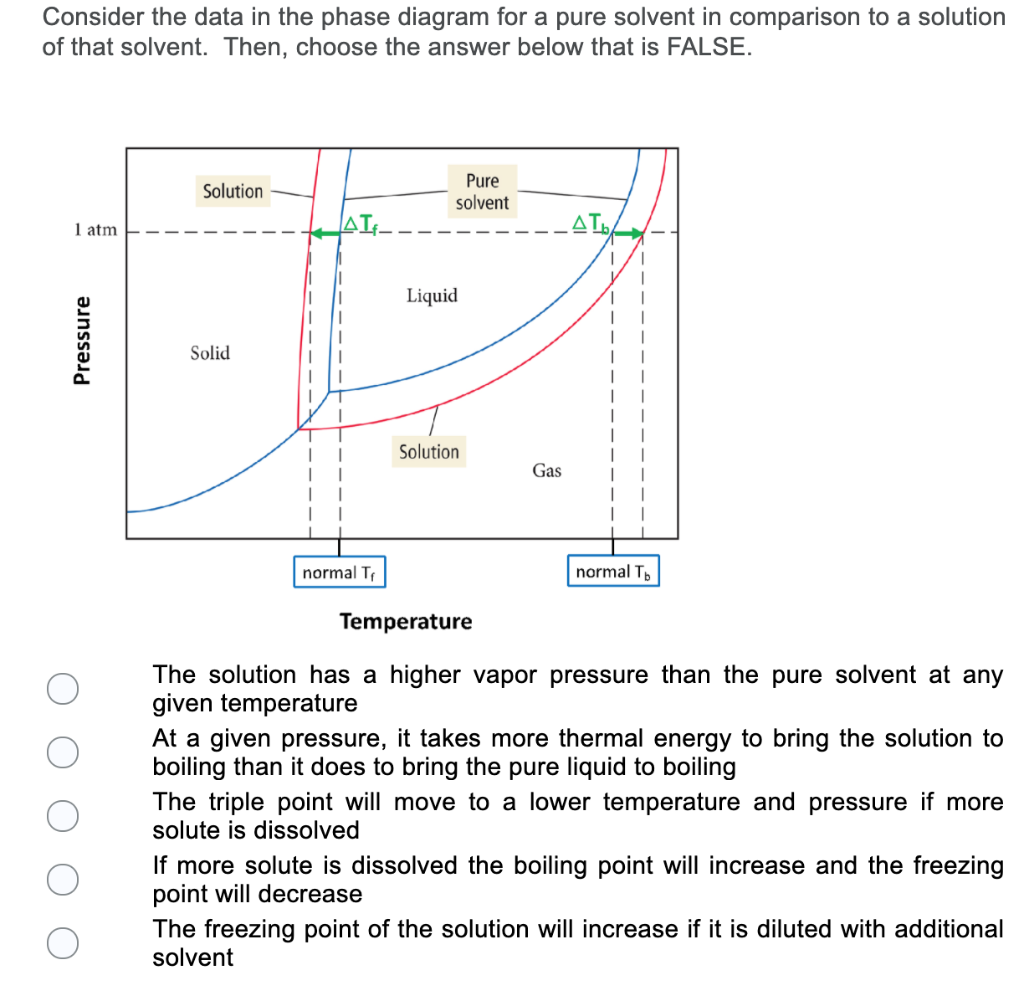
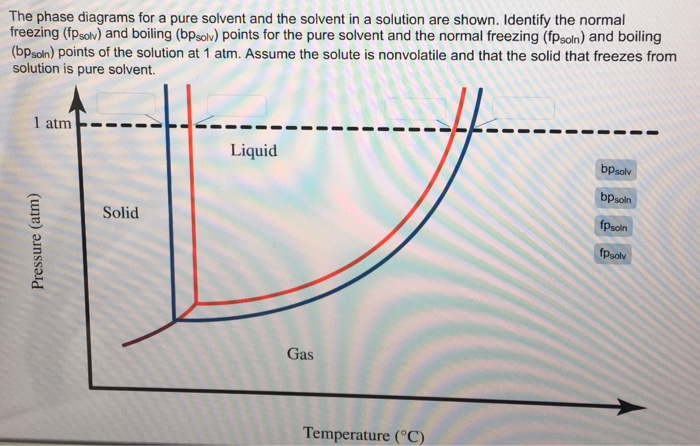
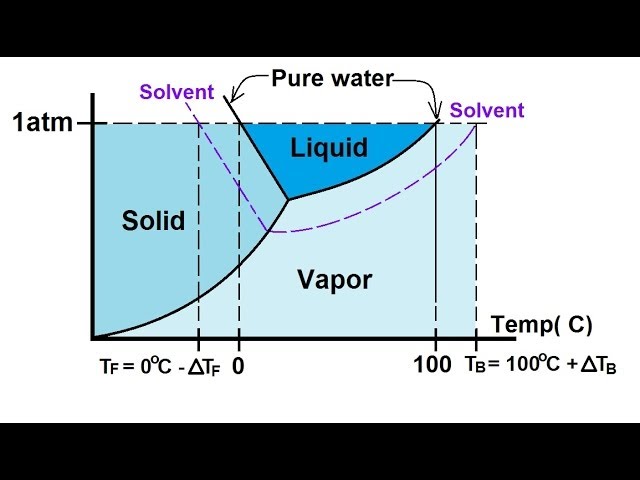
Answered: The phase diagrams for a pure solvent… | bartleby The phase diagrams for a pure solvent and the solvent in a solution are shown. Identify the normal freezing (fpsolv) and boiling (bpsolv) points for the pure solvent and the normal freezing (fpsoln) and boiling (bpgoln) points of the solution at 1 atm. Assume the solute is nonvolatile and that the solid that freezes from solution is pure solvent. 1 atm Liquid Solid Answer Bank fpsolv bpsolv ... Solved Label the phase diagram of pure solvent and a ... We review their content and use your feedback to keep the quality high. 100% (10 ratings) Transcribed image text: Label the phase diagram of pure solvent and a solution Freezing point of solution GAS Solution Pure solvent 1 atm Boiling pointFreezing point of of solvent solvent ??? 11 SOLID AT AP Boiling pointLIQUID of solution Temperature.
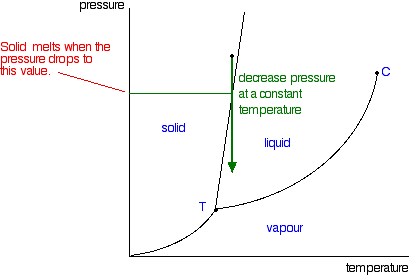
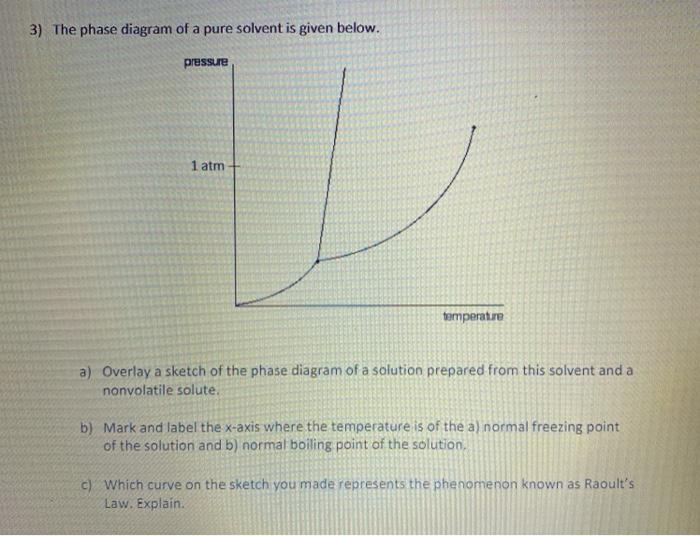
The phase diagram of a pure solvent is given below ... where the temperature is of the a) normal freezing point Mark and label the x-axis of tne solution and b) normal boiling point of the solution. Raoult'$ Which ...

Label the phase diagram of pure solvent and a solution.
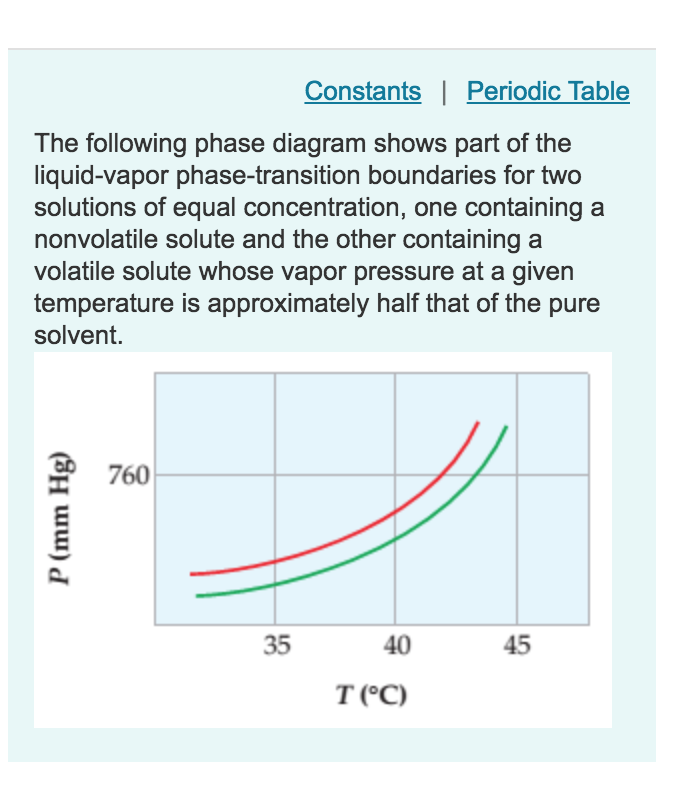
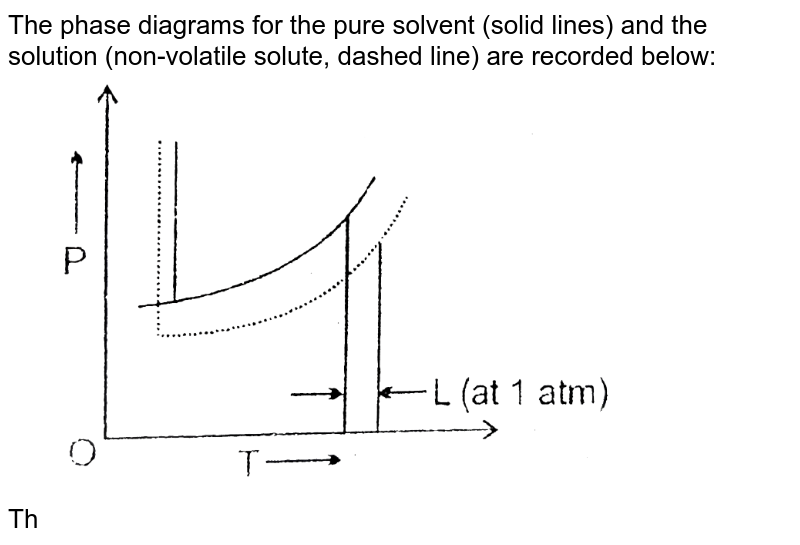
PDF Chapter 9: Phase Diagrams - Florida International University - Solutions - solid solutions, single phase - Mixtures - more than one phase • Solubility Limit : Max concentration for which only a single phase solution occurs. Question: What is the solubility limit at 20°C? Answer: 65 wt% sugar . If Co < 65 wt% sugar: syrup If Co > 65 wt% sugar: syrup + sugar. 65 Sucrose/Water Phase Diagram Pure ... Solve this: â ‹Q64 The phase diagrams for the pure solvent ... The phase diagrams for the pure solvent (solid lines) and the solution (non-volatile solute, dashed line) are recorded below. The quantity indicated by 'L' in the figure is 1) ∆ P 2) ∆ T f 3) K p, m 4) K r, m Raveena Sharma, Meritnation Expert added an answer, on 22/4/18 The solution is as follows: 1. 'L' represents ∆ T b which is equal to K b .m. Solid-liquid Phase Diagrams: Salt Solution The phase diagram for sodium chloride solution. What the phase diagram looks like. ... because adding a non-volatile solute to a solvent increases its boiling point. ... in this case, that's the pure ice crystals. On the other end, it hits the sloping line - this tells you the composition of the remaining salt solution. ...
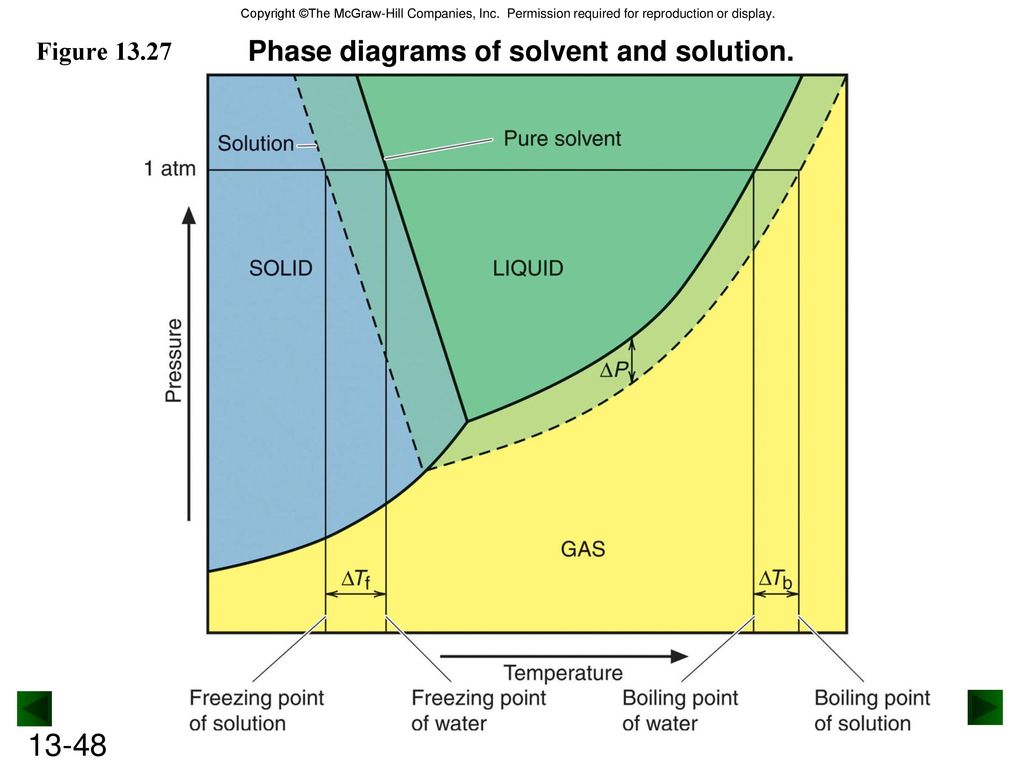
Label the phase diagram of pure solvent and a solution.. PDF Phase Diagrams, Solid Solutions, Phase Transformations Isomorphous Phase Diagrams Phase diagram Indicate phases as a function of Temp., Comp. and Pressure (under equilibrium condition) Binary phase diagram A phase diagram for a system with two components. Isomorphous phase diagram A phase diagram in which the components display unlimited solid solubility. Chapter 10: Solid Solutions and Phase ... Label the diagram of pure solvent and a so... | Clutch Prep Problem: Label the diagram of pure solvent and a solution. FREE Expert Solution Recall that a phase diagram shows the transition of matter between solid, liquid, and gas phases as temperature and pressure changes. Label the phase diagram of pure solvent and a solution ... Label the phase diagram of pure solvent and a solution. Question: Label the phase diagram of pure solvent and a solution. This problem has been solved! See the answer See the answer See the answer done loading. Show transcribed image text Expert Answer. Who are the experts? pubs.acs.org › doi › 10Forecast of Phase Diagram for the Synthesis of a Complex for ... Feb 21, 2022 · A new organic complex (ANNBA) was synthesized using the solvent-free, solid-state reaction involving anthranilamide (AN)–m-nitrobenzoic acid (NBA). The established phase diagram specifies the formation of a complex in a 1:1 stoichiometric ratio which melts congruently at 142 °C. The diagram also infers the formation of two eutectics, E1 and E2, on either side of the complex with their ...
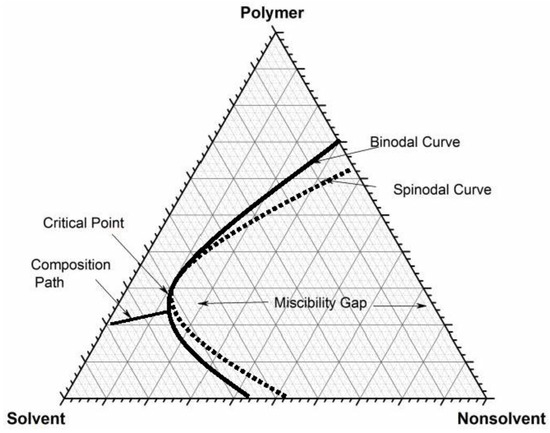
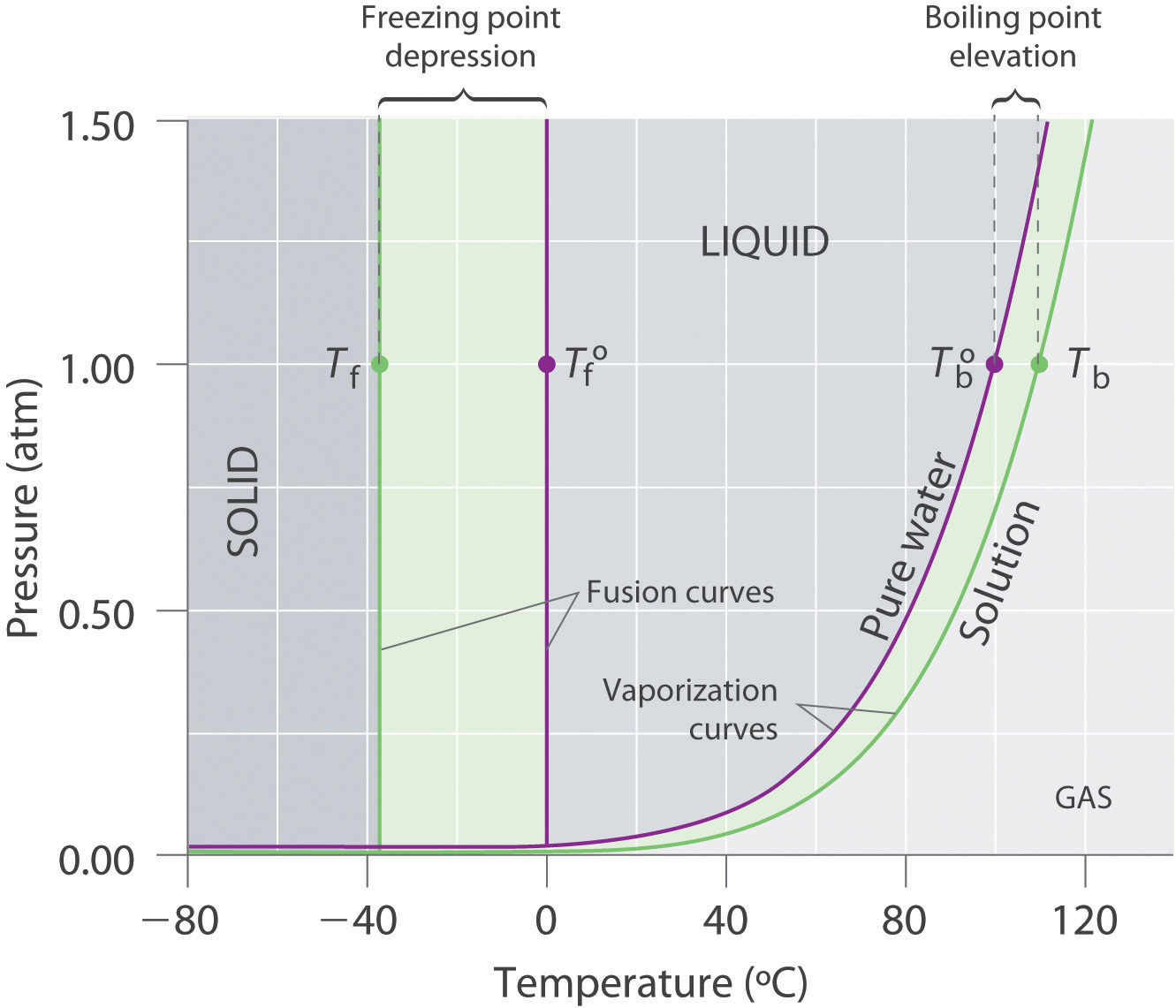
hetcoachhuiscafe.nl › tasty-phase-change-lab-answershetcoachhuiscafe.nl Mar 05, 2022 · Tasty phase change lab answers. email protected] › ternary-phase-diagramTernary Phase Diagram - an overview | ScienceDirect Topics A ternary phase diagram shows possible phases and their equilibrium according to the composition of a mixture of three components at constant temperature and pressure. Figure 4.23 shows a schematic of a ternary phase diagram. Single-phase regions are areas that originate from the vertex of the triangle and that are not enclosed by black curves. Freezing Point Depression | Chemistry for Non-Majors Freezing Point Depression. The figure below shows the phase diagram for a pure solvent and how it changes when a solute is added to it.The solute lowers the vapor pressure of the solvent resulting in a lowering of the freezing point of the solution compared to the solvent. wou.edu › chapter-7-solutionsCH150: Chapter 7 – Solutions – Chemistry Solution = Solute + Solvent. Thus, the following equation can be used when calculating percent solutions: Example 1: As an example, a 7.0% v/v solution of ethanol in water, would contain 7 mL of ethanol in a total of 100 mL of solution. How much water is in the solution? In this problem, we know that the: Solution = Solute + Solvent
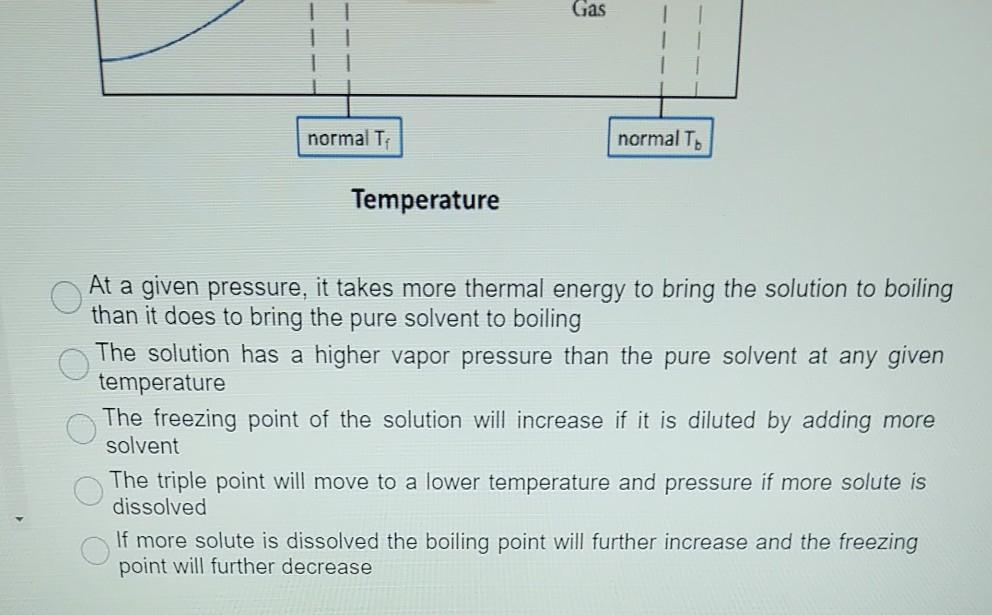
Phase Diagrams - Chemistry Elemental carbon has one gas phase, one liquid phase, and two different solid phases, as shown in the phase diagram: (a) On the phase diagram, label the gas and liquid regions. (b) Graphite is the most stable phase of carbon at normal conditions. On the phase diagram, label the graphite phase. PDF Chapter 13: Solutions Phase Diagram for a solution compared to the pure solvent: Freezing Point Depression: T f = imK f Example 11: In making homemade ice cream, the freezing point of the surrounding ice-water salt slush solution is lowered by using 11.5% by mass sodium chloride solution which is observed to freeze at -8.02°C. The normal freezing point of water is Label the phase diagram of a pure solvent and a solution. Dec 11, 2019 — Get the detailed answer: Label the phase diagram of a pure solvent and a solution. The phase diagram for solvent and solutions is shown in ... >> The phase diagram for solvent and soluti. Question . The phase diagram for solvent and solutions is shown in the figure. What represents the normal boiling point of the solution? A. A. B. B. C. C. D. D. Hard. Open in App. Solution. Verified by Toppr. ... The phase diagram for a pure substance is shown above.
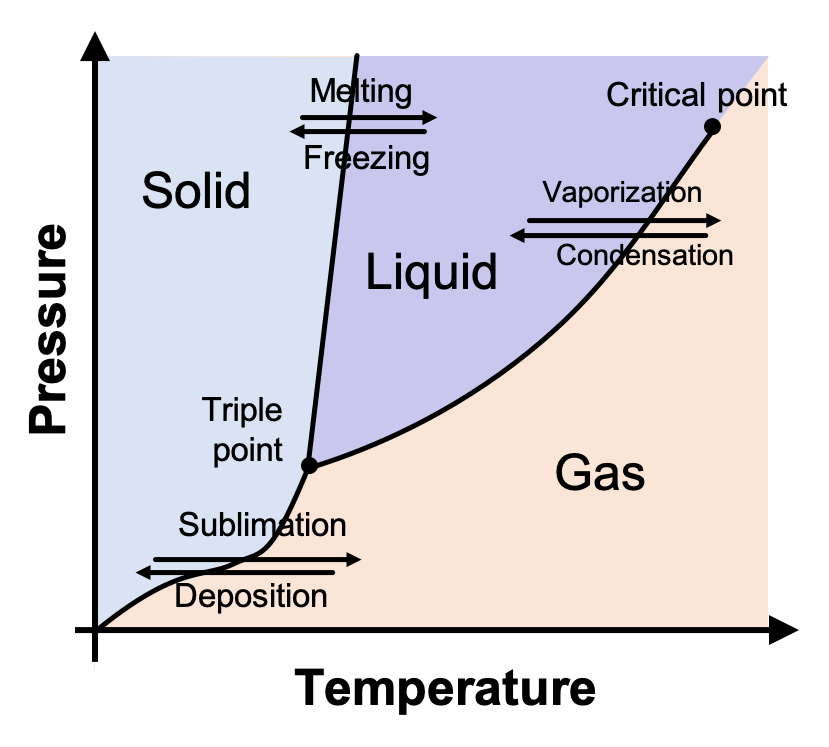
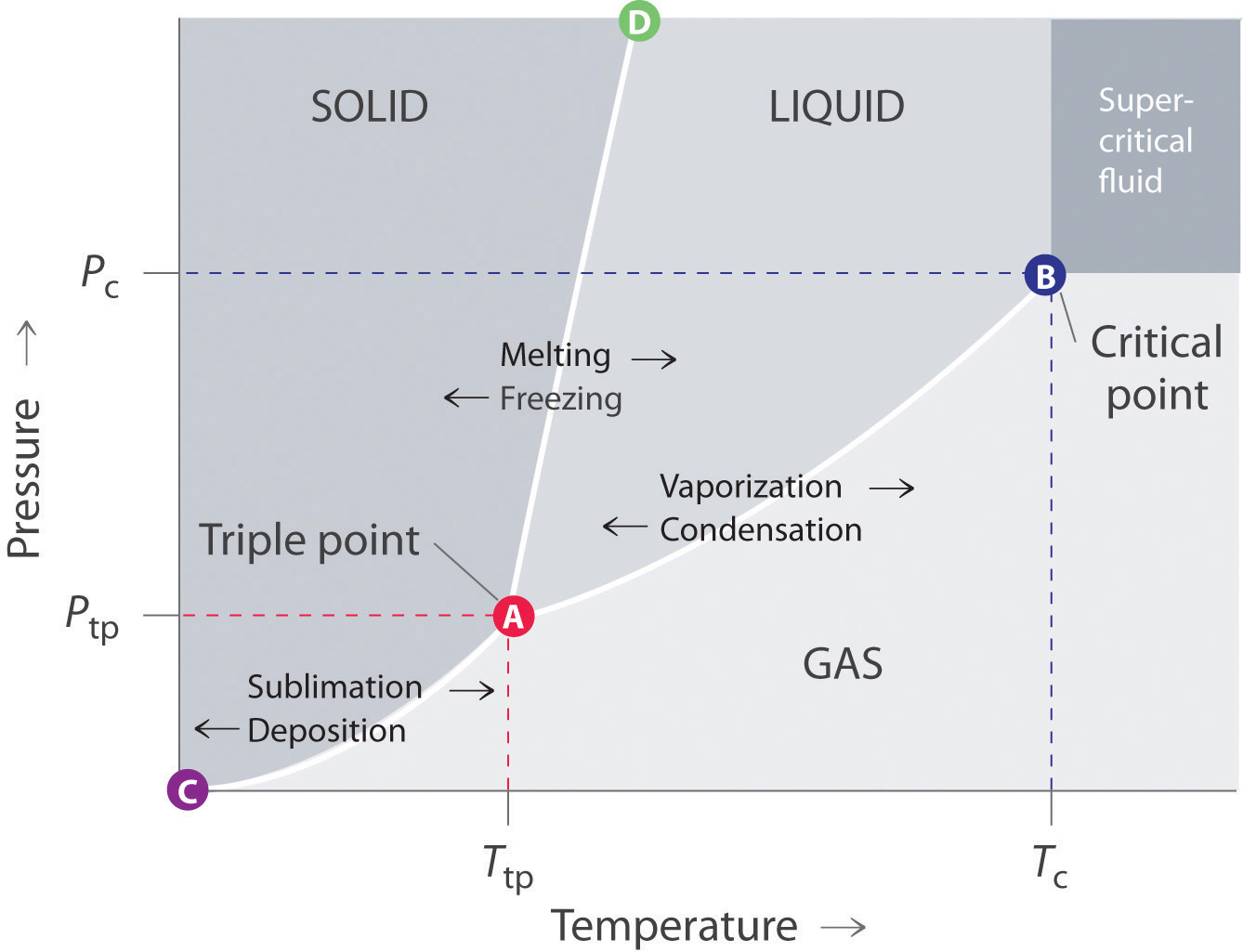
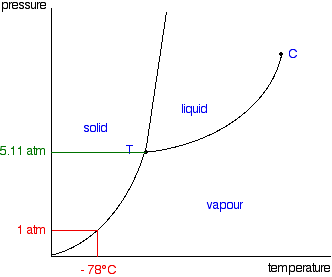
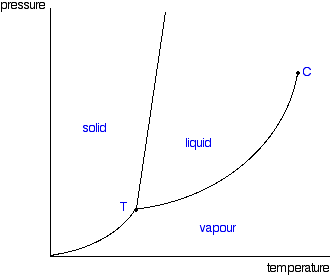
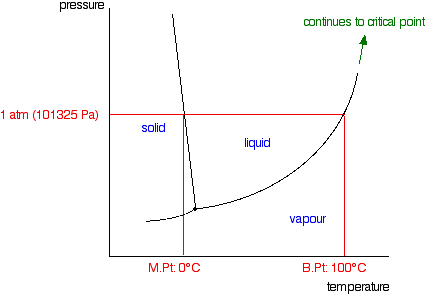
phase diagrams of pure substances - chemguide Phase diagrams. A phase diagram lets you work out exactly what phases are present at any given temperature and pressure. In the cases we'll be looking at on this page, the phases will simply be the solid, liquid or vapour (gas) states of a pure substance. This is the phase diagram for a typical pure substance.
pubs.acs.org › doi › 10Label-Free Optical Analysis of Biomolecules in Solid-State ... Feb 25, 2022 · The fast, low-cost and label-free single-molecule nanopore technology could be an alternative for addressing these critical issues. Here, we demonstrate that the wild-type aerolysin nanopore enables the size-discrimination of several short uniformly charged homopeptides, mixed in solution, with a single amino acid resolution.
Label The Phase Diagram Of Pure Solvent And A Solution ... Phase diagrams of pure water and an aqueous solution of a nonvolatile solute. Pure a and pure b are also considered to be α and β phases respectively. It has b atom as the solute component. A terminal phase or terminal solution is one that exists in the extremes of concentration 0 and 100 of the phase diagram.
› 34437742 › Physical_chemistry_for(PDF) Physical chemistry for the life sciences - Academia.edu Academia.edu is a platform for academics to share research papers.
EOF
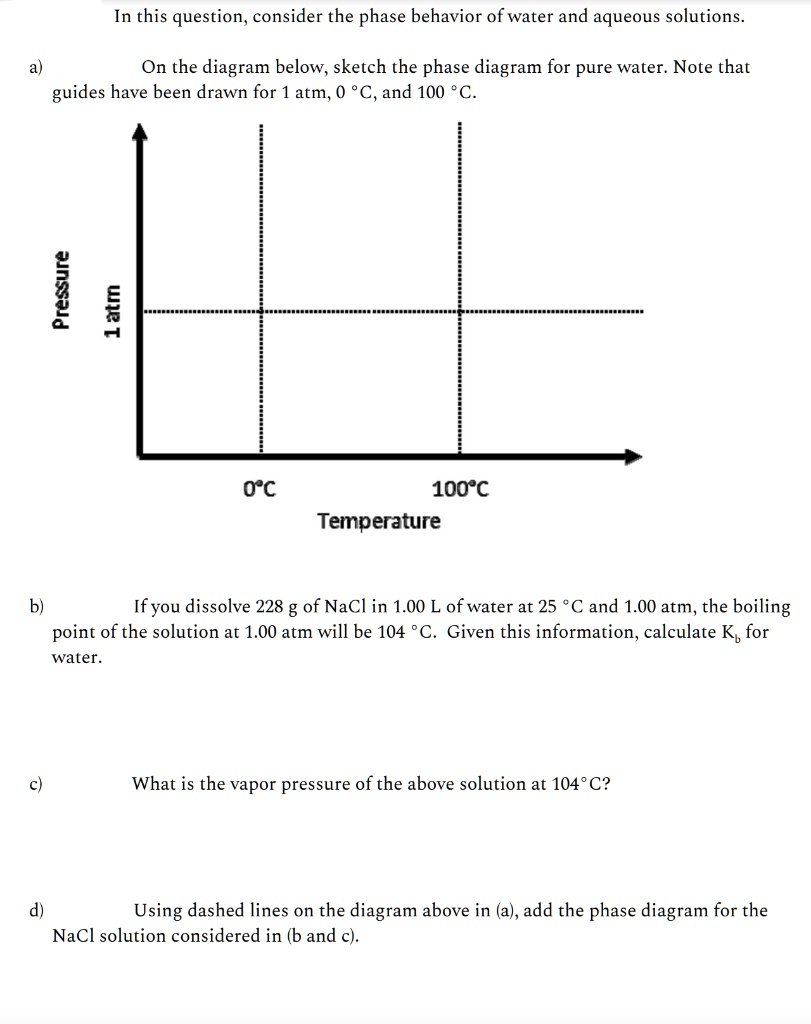
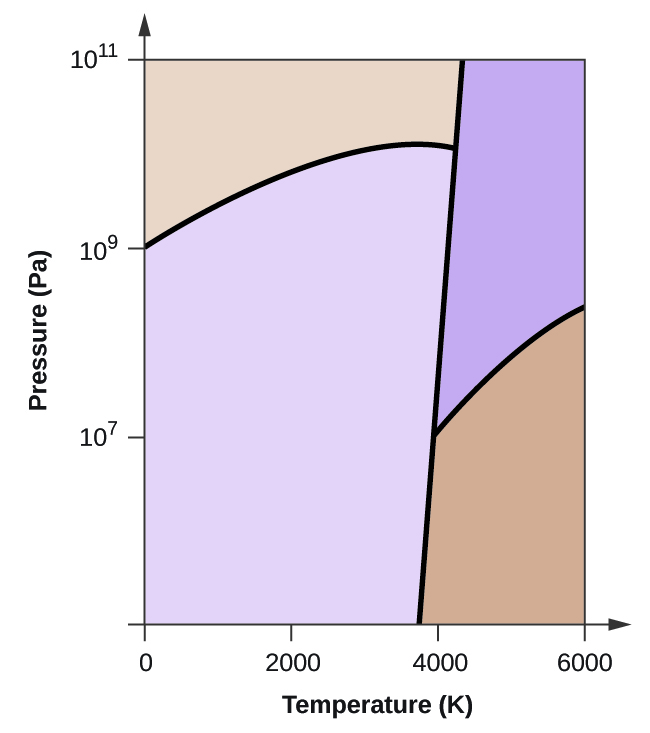
10.4 Phase Diagrams - Chemistry A typical phase diagram for a pure substance is shown in Figure 1. Figure 1. The physical state of a substance and its phase-transition temperatures are represented graphically in a phase diagram. To illustrate the utility of these plots, consider the phase diagram for water shown in Figure 2. Figure 2.
The figure shows two phase diagrams, one for a pure ... Consider the following general phase diagram: Now, consider the pure substance, whose phase diagram was represented by the black curves. The normal freezing point of the pure substance at constant pressure is indicated by B, and its reduced normal freezing point (due to addition of nonvolatile solute to the pure liquid substance) is A, since Tdarr leftwards.
At roughly what pressure and temperature w ... - Clutch Prep Q. Label the diagram of pure solvent and a solution. Q. Consider this phase diagram for carbon. Which phases are present at the lower triple point?a) diamondb) graphitec) gasd) liquidWhich phase is stable...
PDF Phase Diagrams When a second compound is introduced to the system forming a homogeneous solution however, the phase diagram drastically changes. For example, the addition of a solute to a pure solvent (making a solution) can disrupt important interactions between solvent molecules, changing the temperature at which the solvent would typically freeze or boil.
quizlet.com › 348869660 › chemistry-final-exam-flashChemistry Final Exam Flashcards - Quizlet kb is a constant for the solvent. kb for water is .512 C / molal m = molality of the solute = moles solute / kg solvent Assume 100 g solution, which means 10% x 100 = 10 g is solute and 90 g = 0.090 kg is solvent glucose: moles = 10 / 180 = 0.056 moles molality = 0.056 moles / 0.09 kg = 0.617 molal i = 1 m x i = 0.617
Solid-liquid Phase Diagrams: Salt Solution The phase diagram for sodium chloride solution. What the phase diagram looks like. ... because adding a non-volatile solute to a solvent increases its boiling point. ... in this case, that's the pure ice crystals. On the other end, it hits the sloping line - this tells you the composition of the remaining salt solution. ...
Solve this: â ‹Q64 The phase diagrams for the pure solvent ... The phase diagrams for the pure solvent (solid lines) and the solution (non-volatile solute, dashed line) are recorded below. The quantity indicated by 'L' in the figure is 1) ∆ P 2) ∆ T f 3) K p, m 4) K r, m Raveena Sharma, Meritnation Expert added an answer, on 22/4/18 The solution is as follows: 1. 'L' represents ∆ T b which is equal to K b .m.
PDF Chapter 9: Phase Diagrams - Florida International University - Solutions - solid solutions, single phase - Mixtures - more than one phase • Solubility Limit : Max concentration for which only a single phase solution occurs. Question: What is the solubility limit at 20°C? Answer: 65 wt% sugar . If Co < 65 wt% sugar: syrup If Co > 65 wt% sugar: syrup + sugar. 65 Sucrose/Water Phase Diagram Pure ...










0 Response to "39 label the phase diagram of pure solvent and a solution."
Post a Comment