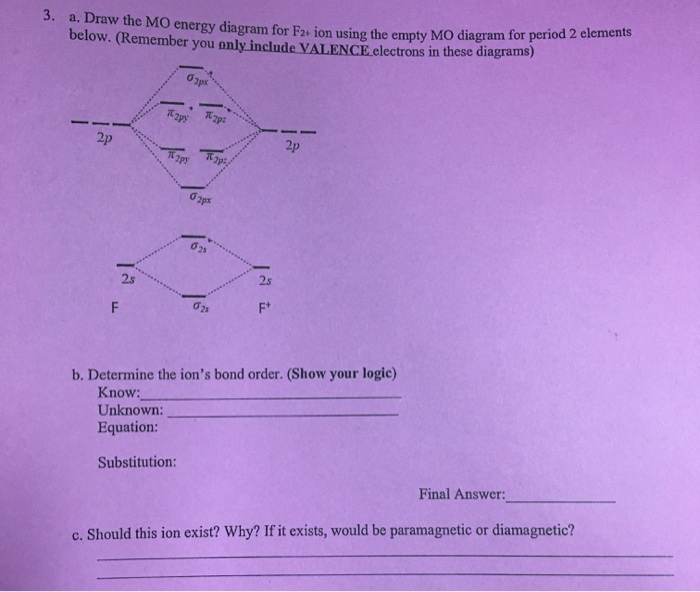
39 f2+ molecular orbital diagram
Energy level diagram for Molecular orbitals - Chemical ... Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons . Number of electrons present in the bonding orbitals is represented by N b and the number of electrons present in antibonding orbitals by Na.. 1) If N b > Na,the molecule is stable because greater number of bonding orbitals are occupied than ... What is an (F2-) bond order? - Quora Answer (1 of 5): The atomic number of fluorine is 9, so a (neutral) F2 molecule has a total of 18 electron, or 14 valence electrons (excluding the four 1s electrons). The (F2)- ion has one more valence electron, or 15. The orbital diagram for a diatomic molecule is To find the bond order, add th...
Member Groups | Institute Of Infectious Disease and ... Three multi-investigator groups that operate principally in the TB/HIV space: The South African TB Vaccine Initiative (SATVI), which includes Mark Hatherill (Director), Tom Scriba (Deputy Director) and Elisa Nemes; The Wellcome Centre for Infectious Diseases Research in Africa (CIDRI-Africa) which includes Robert Wilkinson (Director), Graeme Meintjes, Catherine Riou and Anna …
F2+ molecular orbital diagram
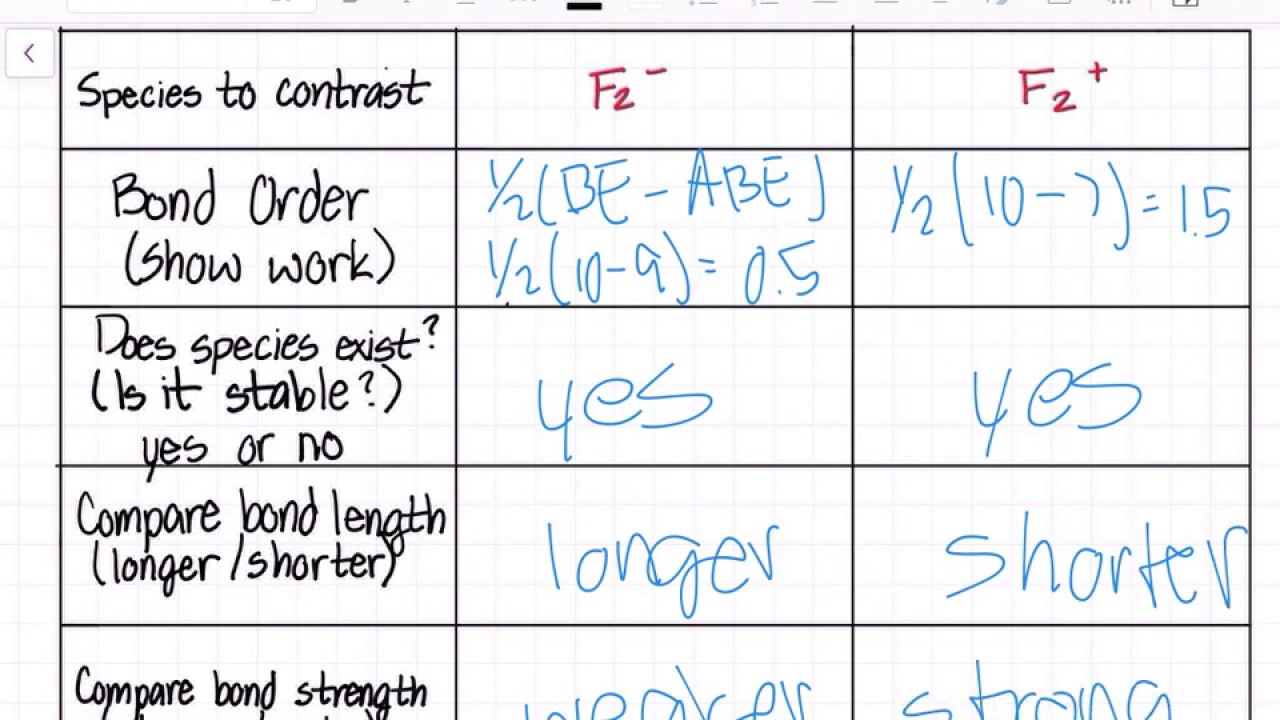
Molecular Orbital (MO) Diagram for F2(2+) - YouTube When two fluorine atoms bond, the sigma(2p) bonding molecular orbitals are lower in energy than the pi(2p) bonding orbitals.F2(2+) has a bond order of 2, so ... Answered: a. Using the molecular orbital diagram,… | bartleby a. Using the molecular orbital diagram, calculate the bond order of F2+. Show show your work or give a brief explanation of the process. b. Do you expect this to have a shorter or longer bond length than F2? Explain your answer. c. Do you expect F2+ to be paramagnetic or diamagnetic? Explain your answer. Molecular Orbital Diagram For Li2 - schematron.org Energy level diagram for Molecular orbitals. May 25, By Mrs Shilpi Nagpal 9 . It is paramagnetic in nature. 6)Li2. Molecular orbital energy level of Li2.Molecular orbitals of Li 2, Be 2, to F 2 The molecular orbital theory (MO) has been introduced for the diatomic hydrogen molecules.
F2+ molecular orbital diagram. What is the molecular electron configuration of "F"_2 ... #sigma# molecular orbitals are singly-degenerate, and #pi# molecular orbitals are doubly-degenerate. #sigma# molecular orbitals, in principle, get more stabilized upon overlap than #pi# molecular orbitals do. For example, an #ns//ns# overlap for a homonuclear diatomic molecule gives rise to a partial MO diagram like this: F2 Molecular Orbital Diagram - 17 images - what is the ... Mar 17, 2022 · [F2 Molecular Orbital Diagram] - 17 images - programable molecular orbital states of c60 from, no2 molecular orbital diagram untpikapps, what is the energy level diagram of n2 and f2 chemistry, mo bonding in f2 and o2 chemistry libretexts, PDF Molecular orbital DiagraM - Magadh University In principle, To construct MO diagram of a any Molecule, first, set up Schrödinger wave equation for that molecule and then, solve it!!! Solution will involve Linear Combination of Atomic Orbitals which are centred around all of the nuclei in molecule, each defined by sets of quantum numbers, with electron probability density determined by ψ 2 Cl2 Molecular Orbital Diagram Click here to get an answer to your question how to drew molecular orbital diagram of Cl2. bond order = 1 (like F2) Cl2 has the weakest bond. b. + would be weaker than in Cl2, the Ar-Ar distance would be molecular orbitals in the diagram suggest. Cl atom has 17 electrons, so chlorine molecule has (Cl2) has 34 electrons. so, bond order of ...
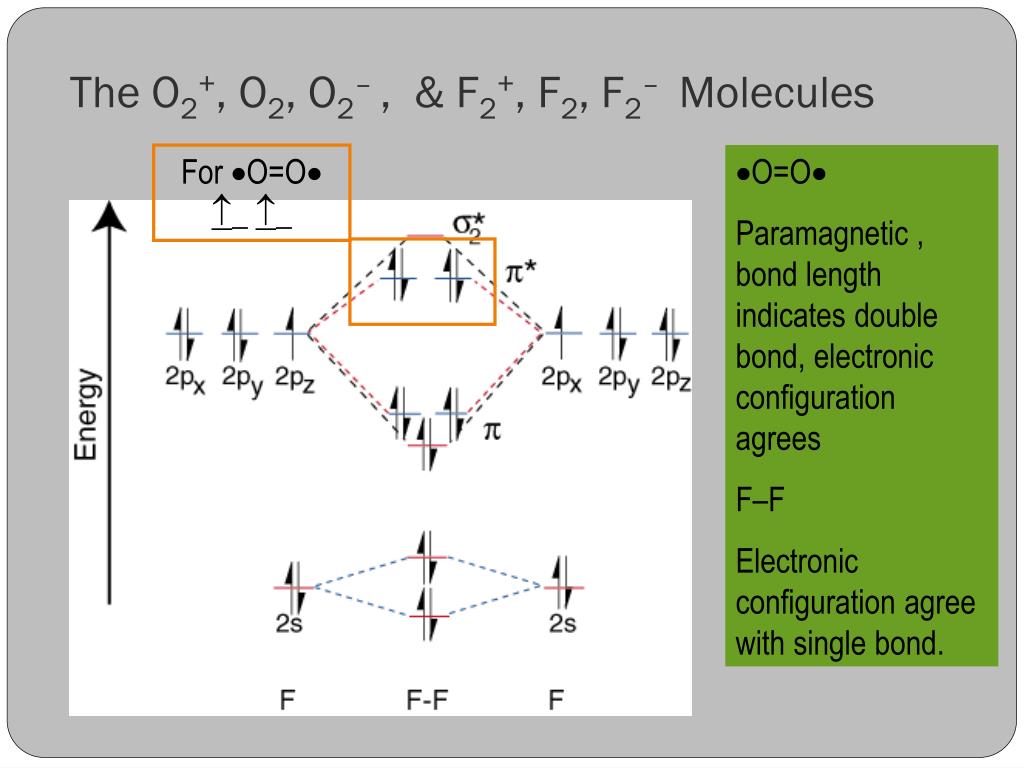
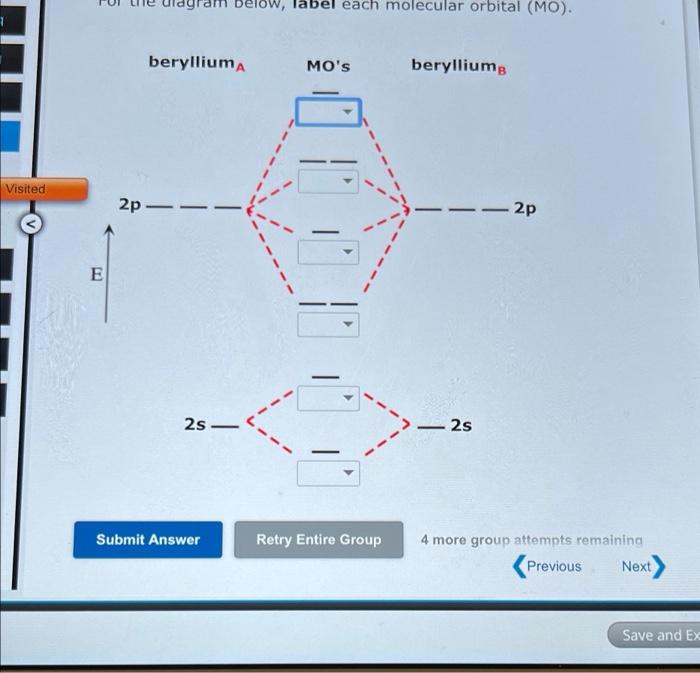
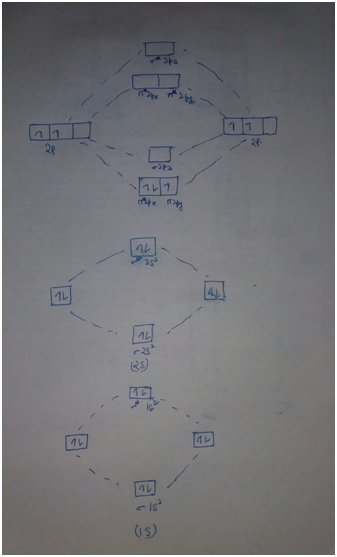
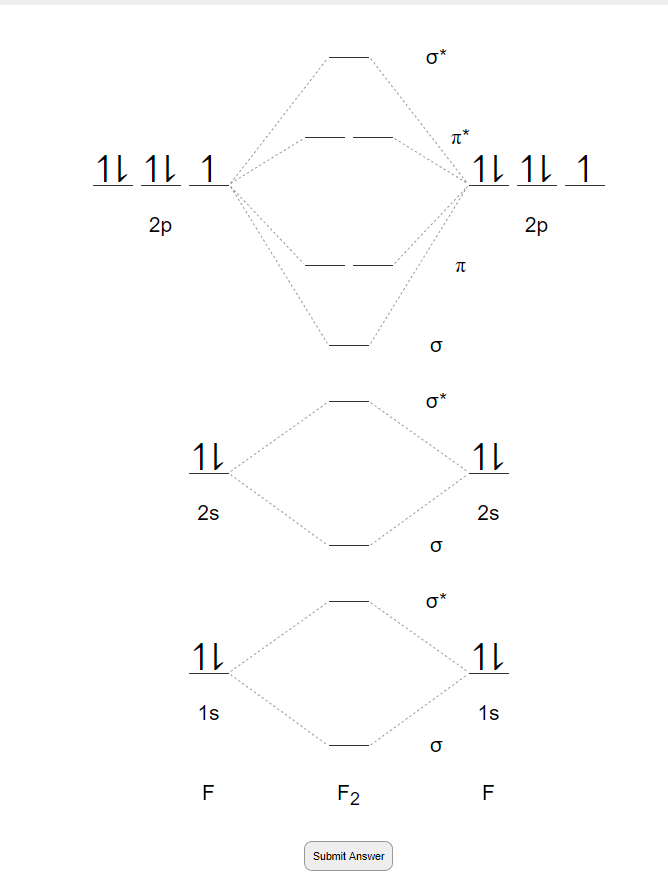
Solved Complete the molecular orbital diagram of F2 and F2 ... Chemistry questions and answers. Complete the molecular orbital diagram of F2 and F2-. What type of orbital contains the highest energy electron (s) in F2? pi, antibonding sigma, bonding sigma, antibonding pi, bonding Which atom is larger in size (radius), Cr or Cr3+? Question: Complete the molecular orbital diagram of F2 and F2-. Draw and write the molecular configuration of nitrogen ... Click here👆to get an answer to your question ️ 37. Draw molecular orbital diagram for F2 molecule. Also, give its electronic configuration, bond order and magnetic property. 138. Solve the following: Molecular Orbital Theory - Chemistry The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. The net contribution of the electrons to the bond strength of a molecule is identified by determining the bond order that results from the filling of the molecular orbitals by electrons. PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
Molecular Orbital Diagram Ne2 - schematron.org Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways. Page 1. MO Diagrams for Elements Li2 through Ne2. (Don't memorize.) Li2 through N2. O2 through Ne2. Use the molecular orbital diagram shown to determine which ... In Molecular orbital diagram, we just need to calculate the number of electrons in anti-bonding orbital and bonding orbital, then we can use the formula in order to calculate bond order is: Bond order = (No. of electrons in anti-bonding MO) - (No. of electrons in bonding MO) / 2 Hope this helps! Molecular Orbital Diagram For Ne2 Figure A partial molecular orbital energy-level diagram for the HF molecule. This interaction introduces an element of s-p mixing, or hybridization, into the molecular orbital theory. The result is a slight change in the relative energies of the molecular orbitals, to give the diagram shown in the figure below. [Expert Answer] Draw the molecular orbital diagram for F2 ... Molecular orbital diagram and bond order of fluorine molecule Fluorine molecule is formed by the combination of atomic orbitals of two fluorine atoms, each having nine electrons, thus making 18 electrons.
PDF Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals.
Solved Question 11 a. Complete the molecular orbital ... Question 11 a. Complete the molecular orbital diagram for F2. Do not include the inner shell electrons. b. Count the number of valence electrons in F2. c. What is the bond order in F2? d. What is the bond order in F2+? e. What is the bond order in F2-? f. Which of the species F2, F2+, or Question: Question 11 a.
Molecular Orbital Theory Diagram Of F2 - The 31 Best ... The 31 best 'Molecular Orbital Theory Diagram Of F2' images and discussions of March 2022. Trending posts and videos related to Molecular Orbital Theory Diagram Of F2!
spectral classification of stars - h a n d p r i n t The spectra show strong molecular bands due to CH, CN, C 2 and in cooler stars SiC 2 and C 3; nearly all the oxygen is bound as CO (carbon monoxide), so there is little left to form other metal oxides such as TiO. Most of the known examples in the visual spectrum are giant stars because nearly all the luminosity is in the infrared and can be ...
Arrange the following in order of decreasing stability. a ... In Molecular orbital diagram, we just need to calculate the number of electrons in anti-bonding orbital and bonding orbital, then we can use the formula in order to calculate bond order is: Bond order = (No. of electrons in anti-bonding MO) - (No. of electrons in bonding MO) / 2 Hope this helps!
Draw molecular orbital diagram for F2 molecule Also class ... 101.4k + views. Hint: The Molecular Orbital Theory (MOT) explains the formation of the molecule in a better way than Valence Bond Theory (VBT). The bond order calculations are feasible using MOT and so is the description of electronic configuration. Thus, the magnetic property can be explained when we know electronic configuration of molecules.
F2 Lewis Structure, Molecular Geometry, Hybridization ... The Lewis theory of chemical bonding helps us visualize the arrangement of atoms—how they are attached or bonded—in molecules. The valence electrons in each atom are the ones that participate in the bonding, and hence they are the only ones displayed in the Lewis structures. It is to be noted though that this theory about the electronic structure is quite primitive and most limited. In a typical Lewis structure, each valence electron is represented as a dot, and a covalent bond between two atoms (formed as a result of sharing of two electrons) is represented as a line. Several atoms tend to seek eight electrons in their valence shell through chemical bonding; this is referred to as the octet rule and is reflected in the Lewis structure of a molecule. Hydrogen is an exception, though; it seeks a duplet, not octet, because it has only one electron in its K shell, and thus needs only one more to achieve the maximum capacity of K shell. Noble gases already have completely filled valance...
What is the molecular orbital diagram of O2 and F2? - Quora Answer (1 of 6): Here is the solution, > * For O2 molecule, > * For F2 molecule, Thanks for reading.
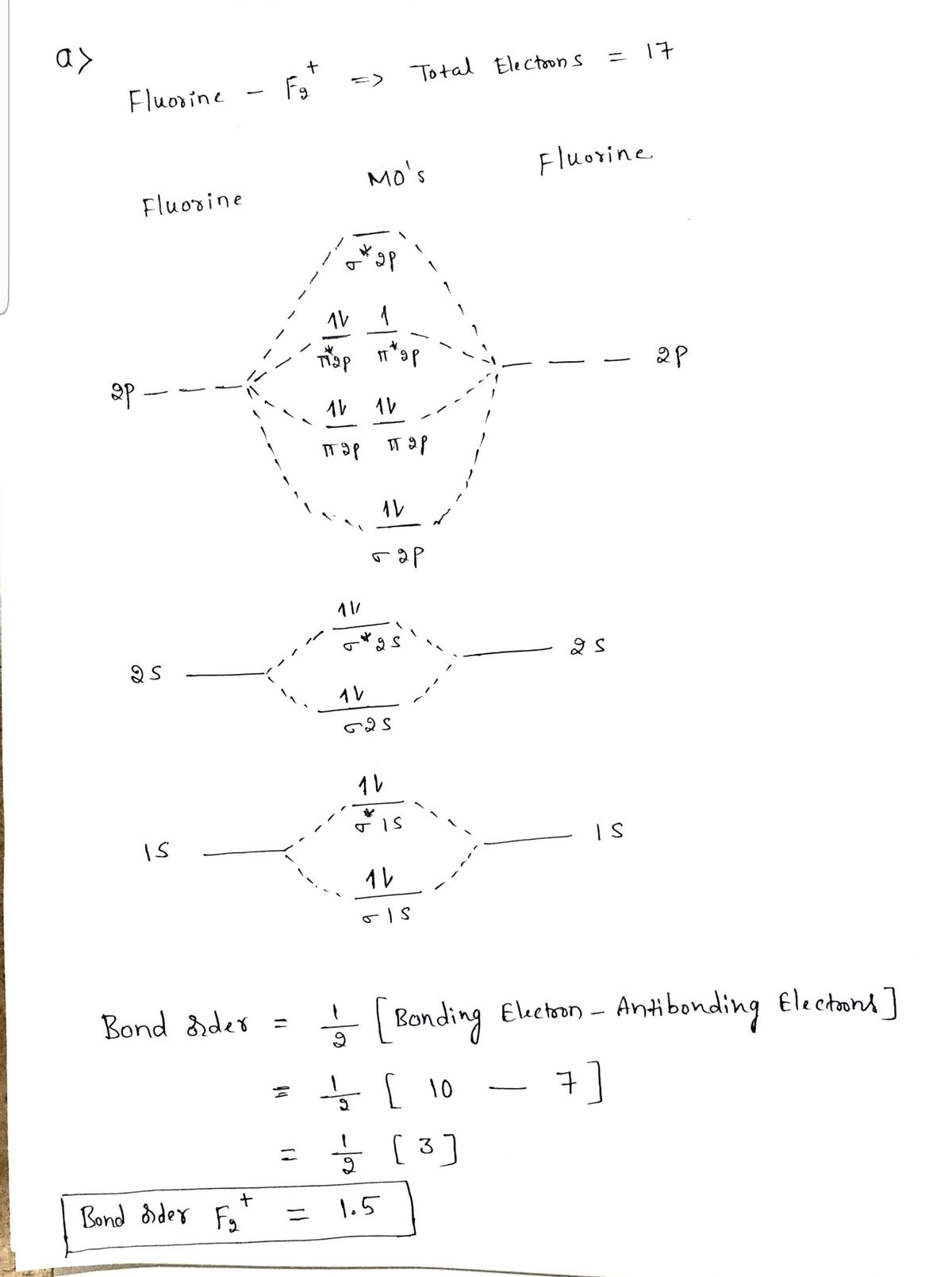
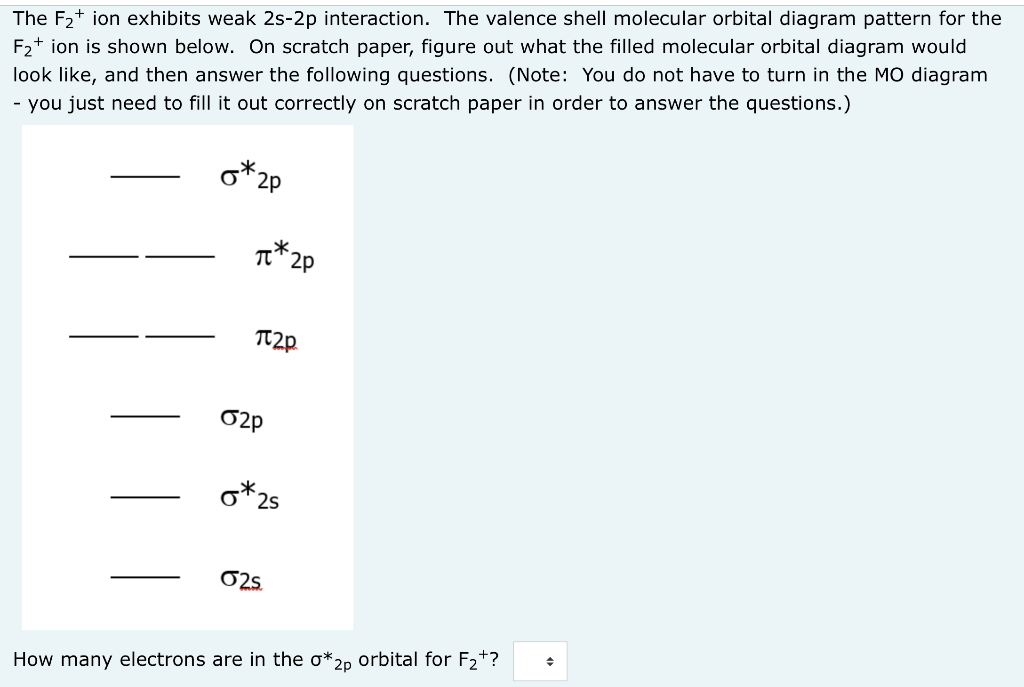
Molecular Orbital Theory - Build F2+ - YouTube For the ion F2+:a) Draw the molecular orbital diagram.b) Calculate the bond order.c) Would this ion exist?d) Write the electron configuration of the ion.————...
F2 Lewis Structure, Molecular Geometry, Hybridization ... 26/03/2022 · F2 Molecular Orbital (MO) Diagram. As per molecular orbital (MO) theory, all the constituent atoms in a molecule contribute to the formation of molecular orbitals. These MOs are a linear combination of the atomic orbitals. Thus, the electrons in a molecule are not individually assigned to atomic orbitals but to molecular orbitals. Let us have a ...
draw the molecular orbital energy level diagram for f2 label all atomic and molecular orbitals a what is the bond order for f2 b is it diamagnetic or paramagnetic c do you expect f2 to be st 73663
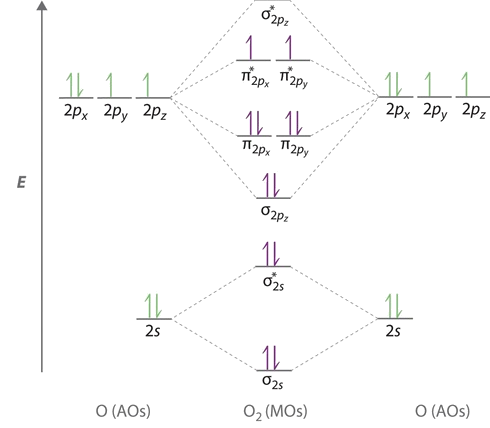
Molecular Orbital Theory - Purdue University diagram shown in the figure below. Experiments have shown that O2and F2are best described by the model in the figure above, but B2, C2, and N2are best described by a model that includes hybridization, as shown in the figure below. Practice Problem 9: Construct a molecular orbital diagram for the O2molecule. Click
Institute Of Infectious Disease and Molecular Medicine For information on South Africa's response to COVID-19 please visit the COVID-19 Corona Virus South African Resource Portal.
Draw a molecular orbital diagram of N2 or O2 with magnetic ... Complete step by step answer: First let us understand the concept of molecular orbital theory. On a very general basis, electrons are not assigned to individual bonds between atoms, but they move under the influence of the nuclei in the whole molecule. Molecular orbital theory is a method for describing the electronic structure of the molecule.
Use the molecular orbital energy level diagram to show ... Click here👆to get an answer to your question ️ Use the molecular orbital energy level diagram to show that N2 would be expected to have a triple bond, F2 , a single bond and Ne2 , no bond.
Molecular Orbital Diagram For Li2 - schematron.org Energy level diagram for Molecular orbitals. May 25, By Mrs Shilpi Nagpal 9 . It is paramagnetic in nature. 6)Li2. Molecular orbital energy level of Li2.Molecular orbitals of Li 2, Be 2, to F 2 The molecular orbital theory (MO) has been introduced for the diatomic hydrogen molecules.
Answered: a. Using the molecular orbital diagram,… | bartleby a. Using the molecular orbital diagram, calculate the bond order of F2+. Show show your work or give a brief explanation of the process. b. Do you expect this to have a shorter or longer bond length than F2? Explain your answer. c. Do you expect F2+ to be paramagnetic or diamagnetic? Explain your answer.
Molecular Orbital (MO) Diagram for F2(2+) - YouTube When two fluorine atoms bond, the sigma(2p) bonding molecular orbitals are lower in energy than the pi(2p) bonding orbitals.F2(2+) has a bond order of 2, so ...



![Solved] Consider the Following Series of Molecular Ions and ...](https://d2lvgg3v3hfg70.cloudfront.net/TB2288/11ea7a3a_9ef5_28a9_a82d_89a59812d74c_TB2288_00.jpg)
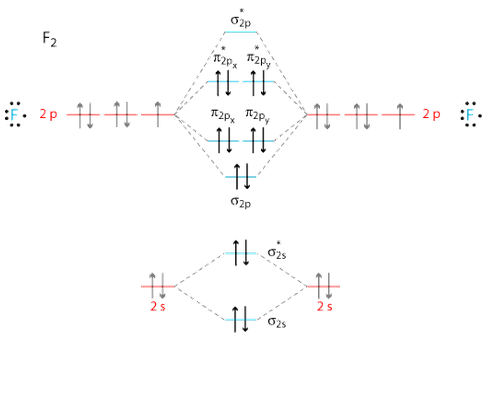
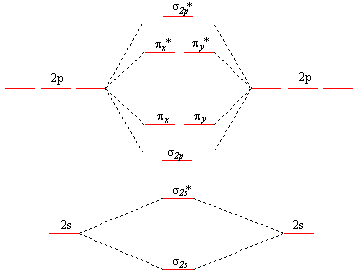









![Expert Answer] Draw the molecular orbital diagram for F2 and ...](https://hi-static.z-dn.net/files/dae/d7baa23a1d4a2ea2c90e0a703e2fd41d.jpg)


0 Response to "39 f2+ molecular orbital diagram"
Post a Comment