38 orbital diagram for cr3+
Chem 2 final Flashcards | Quizlet Cr3+ Which of the following is not true about a spontaneous process? A) A spontaneous process is one that continues on its own once begun. B) Spontaneous processes occur naturally. C) A nonspontaneous process does not occur unless some external action is applied. D) A nonspontaneous process does not mean it cannot ever occur under any conditions. E) A … Chromium(Cr) electron configuration and orbital diagram Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electrons of the atom revolve around the nucleus in a certain circular path. These circular paths are called orbit(shell). These orbits are expressed by n. [n = 1,2,3,4 . . . The serial number of the orbit] K is the name of the first orbit, L is the second, M is the third, N is the name of the fourth orbit. The electron holding capacity of each orbit is 2n2. For example, 1. n = 1 for K orbit. The electron holding capacity of K orbit is 2n2 = 2 × 12= 2 electrons. 2. For L orbit, n = 2. The electron holding capacity of the L orbit is 2n2 = 2 × 22= 8 electrons. 3. n=3 for M orbit. The maximum electron holding capacity in M orbit is 2n2 = 2 × 32 = 18 electrons. 4. n=4 for N orbit. The maximum electron holding capacity in N orbit is 2n2 = 2 × 42= 32 electrons. Therefore, the maximum electron holding capacity in the first shell...
SOLVED:Write orbital diagrams for each ion and determine ... Answer. Write orbital diagrams for each ion and determine if the ion is diamagnetic or paramagnetic. a. V5+ b. Cr3+ c. Ni2+ d. Fe3+
Orbital diagram for cr3+
Mo3+ Orbital Diagram - Wiring Diagrams The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins. The boxes represent sulfur's orbitals. Sulfur's electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 4. Orbital Diagram For V5+ - schematron.org Answer to Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+ Determine if the ion is diamagnetic or paramagnetic. V. Since the 4s orbital is higher in energy, its electrons will be removed first. Not that it matters here, though, because exactly 5 electrons are. SOLVED:Write orbital diagrams for each ion and determine ... take a look here at utilizing the periodic table too. Do some orbital configurations or diagrams for some metal cat ions. So first example is going to be for chromium three plus. So as we follow the periodic table and fill the electrons properly, first row of the periodic table. Um we feel the first two electrons in the structure in the one S sub shell with its one orbital.
Orbital diagram for cr3+. Quantum Number Questions and Answers | Study.com Quantum Number Questions and Answers. Get help with your Quantum number homework. Access the answers to hundreds of Quantum number questions that are explained in a way that's easy for you to ... Answered: Write orbital diagrams for each ion and… | bartleby Write orbital diagrams for each ion and indicate whether the ion is diamagnetic or paramagnetic. a. V5 + b. Cr3 + c. Ni2 + d. Fe3 +. Expert Solution. PDF MO Diagrams for More Complex Molecules • MO diagrams can be built from group orbitals and central atom orbitals by considering orbital symmetries and energies. • The symmetry of group orbitals is determined by reducing a reducible representation of the orbitals in question. This approach is used only when the group orbitals are not obvious by inspection. Orbital Diagram For Strontium - schematron.org In an orbital (box) diagram a box represents each notation and an orbital diagram. strontium atom (a) in the spdf notation and (b) in the. Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of strontium (atomic number: 38), the most. Oxidation States, 2. Electrons Per Shell, 2 8 18 8 2.
Essentials of Physical Chemistry by B.S ... - Academia.edu Preface The Essentials of Physical Chemistry has been written for BSc students. It has been national bestseller for more than 65 years. It has been used by more than 2 million students. It is 26 editions old. It really has been that long. A lot of (PDF) Inorganic Chemistry by Miessler ~ 5th Edition ... This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry. (PDF) Inorganic Chemistry by Miessler ~ 5th Edition | Arnab Patra - Academia.edu What is the electron configuration of Cr 3+? - Get the answer to this question and access a vast question bank that is tailored for students. Login Study Materials BYJU'S Answer NCERT Solutions NCERT Solutions For Class 12 NCERT Solutions For Class 12 Physics NCERT Solutions For Class 12 Chemistry NCERT Solutions For Class 12 Biology Ionic Association in CH3–(CH2–CF2)n–CH3(PVDF)–Li+–(CF3SO2 ... The ionic conductivity of solid polymer electrolytes is governed by the ionic association caused by the polymer···Li+ and the anion···Li+ interactions. We performed the density functional calculation to analyze the molecular interactions in the CH3–(CH2–CF2)n–CH3–Li+–(CF3SO2)2N– for n = 1,4 systems. The gauche conformation is predicted in the lowest energy conformer of pure ...
Complete Solutions Manual General Chemistry Ninth Edition ... Complete Solutions Manual General Chemistry Ninth Edition ... - ID:5dcdb97adce08. Complete Solutions Manual GENERAL CHEMISTRY NINTH EDITION Ebbing/Gammon. Uploaded by. Sofia Uribe Sanchez. connect to do... Solved Part B Enter an orbital diagram for Cr3+. Drag the ... Best Answer. This is the best answer based on feedback and ratings. Transcribed image text: Part B Enter an orbital diagram for Cr3+. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Targets may be left blank, such as for unused orbitals. Reset Help 1111s 2s 4s 2p 30 40 30 G1 G1 G1 ... Bromine Orbital Diagram - Wiring Diagrams Bromine Orbital Diagram. Answer to Write the electron configuration and give the orbital diagram of a bromine (Br) atom (Z = 35). the σ bonds. I've drawn the overlaps below in the MO diagrams. Each bromine would donate one 4pz electron to form a σ -bonding orbital. Answer to Draw an orbital diagram for each element: (a) magnesium; (b ... Orbital Diagram For Au+ - schematron.org The atomic number of Au is Therefore, its For Au+, one electron is removed from the outermost 6s orbital, making the configuration. Electron Configuration, [Xe] 4f14 5d10 6s1. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1. Orbital Diagram. 1s. ↿⇂. 2s. ↿⇂. 2p. Write orbital diagram for Au+? ↿⇂. ↿⇂. ↿⇂.
Problem Set #4 (Ch 3, 4) Flashcards - Quizlet Specify the electron configurations for each of the following ions by developing the orbital diagram. The electron configuration will display below the diagram. O2- Br- Sr2+ Co3+ Cu2+ [He] 2s^2 2p^6 [Ar] 3d^10 4s^2 4p^6 [Ar] 3d^10 4s^2 4p^6 [Ar] 4s^0 3d^6 [Ar] 4s^0 3d^9 Write orbital diagrams for each of these ions. V5+ Cr3+ Ni2+ Fe3+
PDF Electron Configurations and Orbital Diagrams key 1. Describe the two differences between a 2p x orbital and a 3p y orbital. The 2px orbital lies on the x-axis. The 3py orbital lies on the y-axis and is larger than the 2px orbital. 2. The lobes of a p orbital disappear at the nucleus. What does this tell us about electrons in p orbitals?
Chem Lab Quiz Flashcards | Quizlet Cr3+ Ru2+ Cd2+ How many unpaired electrons does Mn2+ contain? 5. On what axis of a graph will the dependent variable appear? Y-axis . What should be included on a graph with more than one dataset that does not appear on a graph with only one data set? Legend. On what axis of a graph will the independent variable appear? X-axis. How many graphs will you prepare as part …
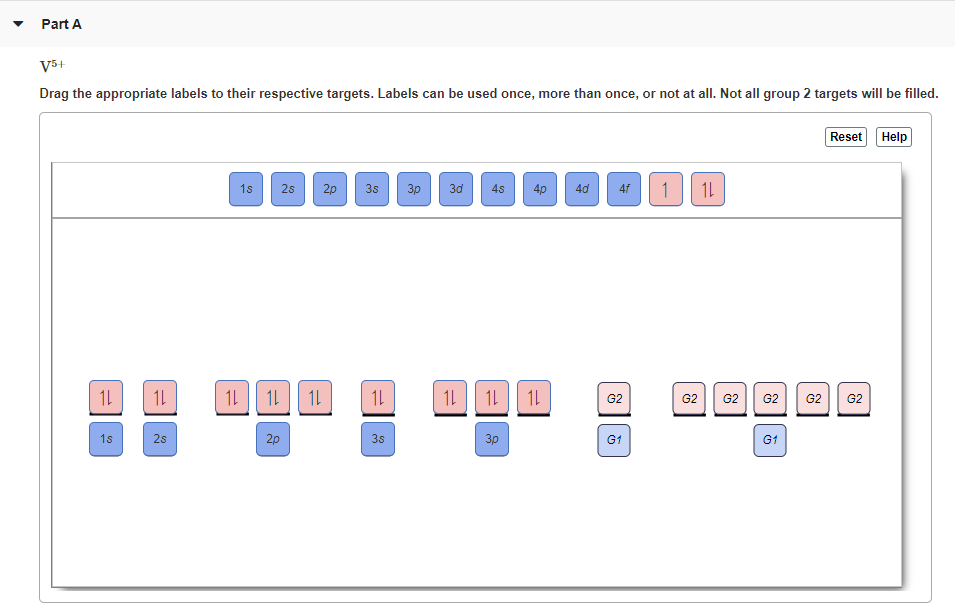
Solved Part A Enter an orbital diagram for V5+ Drag the ... Expert Answer Transcribed image text: Part A Enter an orbital diagram for V5+ Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all.
Electron Configuration for Chromium (Cr, Cr2+, Cr3+) Electron Configuration for Cr, Cr2+, and Cr3+ (Exception to Rules) In writing the electron configuration for Chromium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Chromium go in the 2s orbital. The next six electrons will go in the 2p orbital.
Chapter 7: Quantum Theory and the Electronic Structure of ... The orbital diagram for a ground state carbon atom is. 15. Which ground-state atom has an electron configuration described by the following orbital diagram? A) phosphorus B) germanium C) selenium D) tellurium E) none of these ... Write the ground-state electron configuration for Cr3+. 94. Write the ground-state electron configuration for Ni2 ...
Orbital Diagram For Fe3+ Orbital Notation is a way to show how many electrons are in anorbital for a given element. They can either be shown with arrowsor circles. One arrow represents one electron in a shell. Twoarrows will be pointing differently; one up and one down to show amaximum of two electrons with different spin.
Half equations quiz questions - Footprints-Science | GCSE ... The lungs Quiz States of matter Quiz Chromatography Quiz GCSE Biology sample animations and quizzes GCSE Chemistry sample animations and quizzes GCSE Physics sample animations and quizzes GCSE Investigative Skills animations/slides
Electron Configuration Questions and Answers - Study.com Cr3+ View Answer. Using an orbital diagram, determine the number of unpaired electrons in selenium. View Answer. Write the electron configuration of the ion formed by cesium. View Answer. Are the ...
Orbital Diagram of All Elements (Diagrams given Inside) Apr 10, 2021 · Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ...
V5+ Orbital Diagram - schematron.org Best wishes, Charlie Grame.So let's look at the Auf Bau diagram which actually show this for us, okay so down here we have the 1s orbital but the 1 dash indicates that there's 1 orbital within the 1s sublevel which makes sense that it is the lowest in energy, it's its first principle energy level. Write orbital diagrams for each of these ions. A.
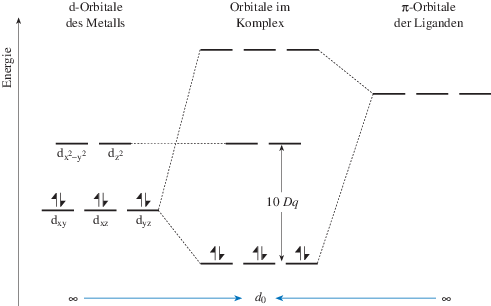
SOLVED:Draw the octahedral crystal field splitting diagram ... Draw the octahedral crystal field splitting diagram for each metal ion. a. Cr3+ b. Cu2+ c. Mn3+ (high- and low-spin) d. Fe2+ (low-spin) Answer a) C r 3 + IMAGE NOT AVAILABLE b) C u 2 + IMAGE NOT AVAILABLE c) M n 3 + IMAGE NOT AVAILABLE d) F e 2 + Low spin IMAGE NOT AVAILABLE View Answer Discussion You must be signed in to discuss.
Write orbital diagrams for each ion and indicate whether ... Step 1. 1 of 6. In writing orbital diagrams, first, determine the electron configuration of the neutral atom and remove electrons accordingly. Diamagnetic ions contain no unpaired electrons. Paramagnetic ions have unpaired electrons. Determine the orbital diagrams of a. V. 5 + ^ {5+} 5 +. b.
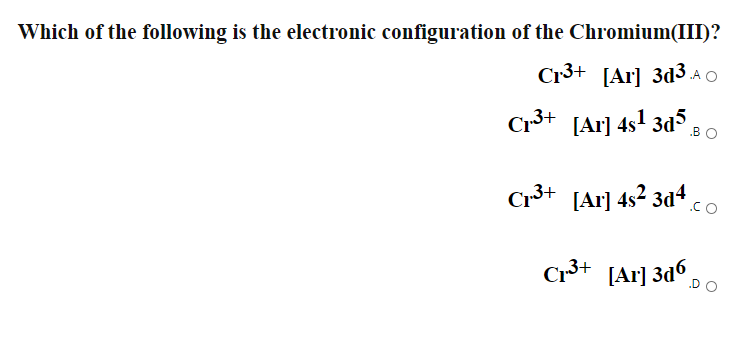
What is the electron configuration of Cr 3+? | Socratic Nov 30, 2015 · Note that it is 4s13d5 and not 4s23d4 because a half filled d orbital is more stable than a partially filled d orbital. However, the chromium ion Cr3+ possesses 24e− −3e− = 21e− due to the loss of 3 of its electrons. Thus, the electron configuration of Cr3+ is: Cr3+:1s22s22p63s23p64s03d3. Answer link.
PDF Principles of Chemical Science, Solutions for Lecture 28 ... an crystal field splitting diagrams to show orbital occupancies in both weak and strong octahedral fields, and (ii) indicate the number of unpaired electrons in each case. Label . the diagrams (iii) weak or strong field, (iv) high spin or low spin (as appropriate), (v) with the names of the d-orbitals, and (vi) with the appropriate orbital sets ...
PDF Orbital diagram for zinc The 1s orbital at the bottom of the diagram is orbital with electrons of the lowest energy. Zinc (Zn). So the only possible stable configuration would be 4s2 3d3. Fill in the orbital energy diagram for zinc ion The lowest E levels are already filled in for you . Write an orbital diagram for the state of the earth of the zinc atom.
PDF Electron configuration of cr3 Electron configuration of cr3 ... Why are unique external electrons included in the orbital filling diagram? They are the only ones involved in chemical reactions and bonding. 2s Orbital is farther from the nucleus meaning that has more energy. Beginner enthalpy ionization. Entertainment ionization of elements is the quantity of energy that an ...
Electron Configuration for Cr, Cr2+, and Cr3+ (Exception to ... To write the configuration for the Chromium ions, first we need to write the electron configuration for just Chromium (Cr). We first need to find the number...
SOLVED:Write orbital diagrams for each ion and determine ... take a look here at utilizing the periodic table too. Do some orbital configurations or diagrams for some metal cat ions. So first example is going to be for chromium three plus. So as we follow the periodic table and fill the electrons properly, first row of the periodic table. Um we feel the first two electrons in the structure in the one S sub shell with its one orbital.
Orbital Diagram For V5+ - schematron.org Answer to Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+ Determine if the ion is diamagnetic or paramagnetic. V. Since the 4s orbital is higher in energy, its electrons will be removed first. Not that it matters here, though, because exactly 5 electrons are.
Mo3+ Orbital Diagram - Wiring Diagrams The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows represent the 16 electrons of the sulfur atom, and the directions represent their spins. The boxes represent sulfur's orbitals. Sulfur's electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 4.
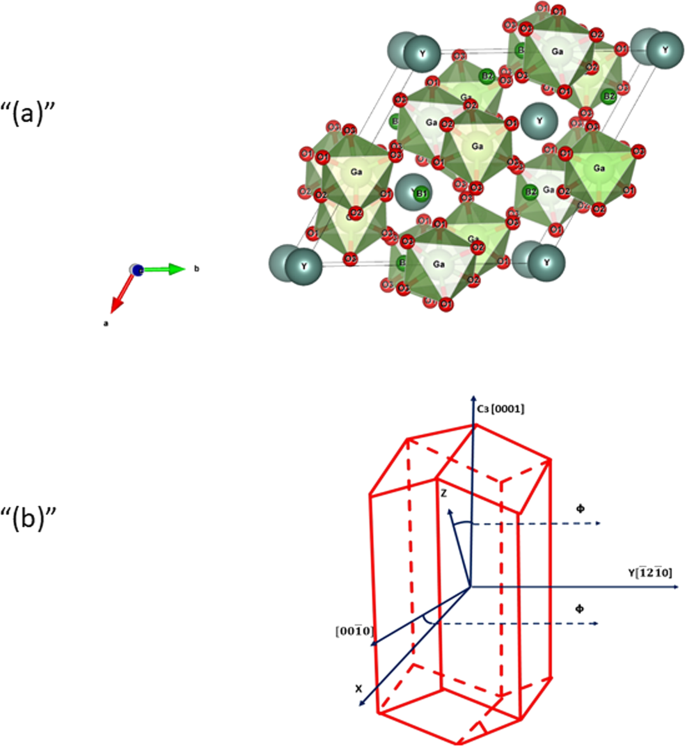
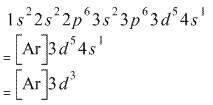







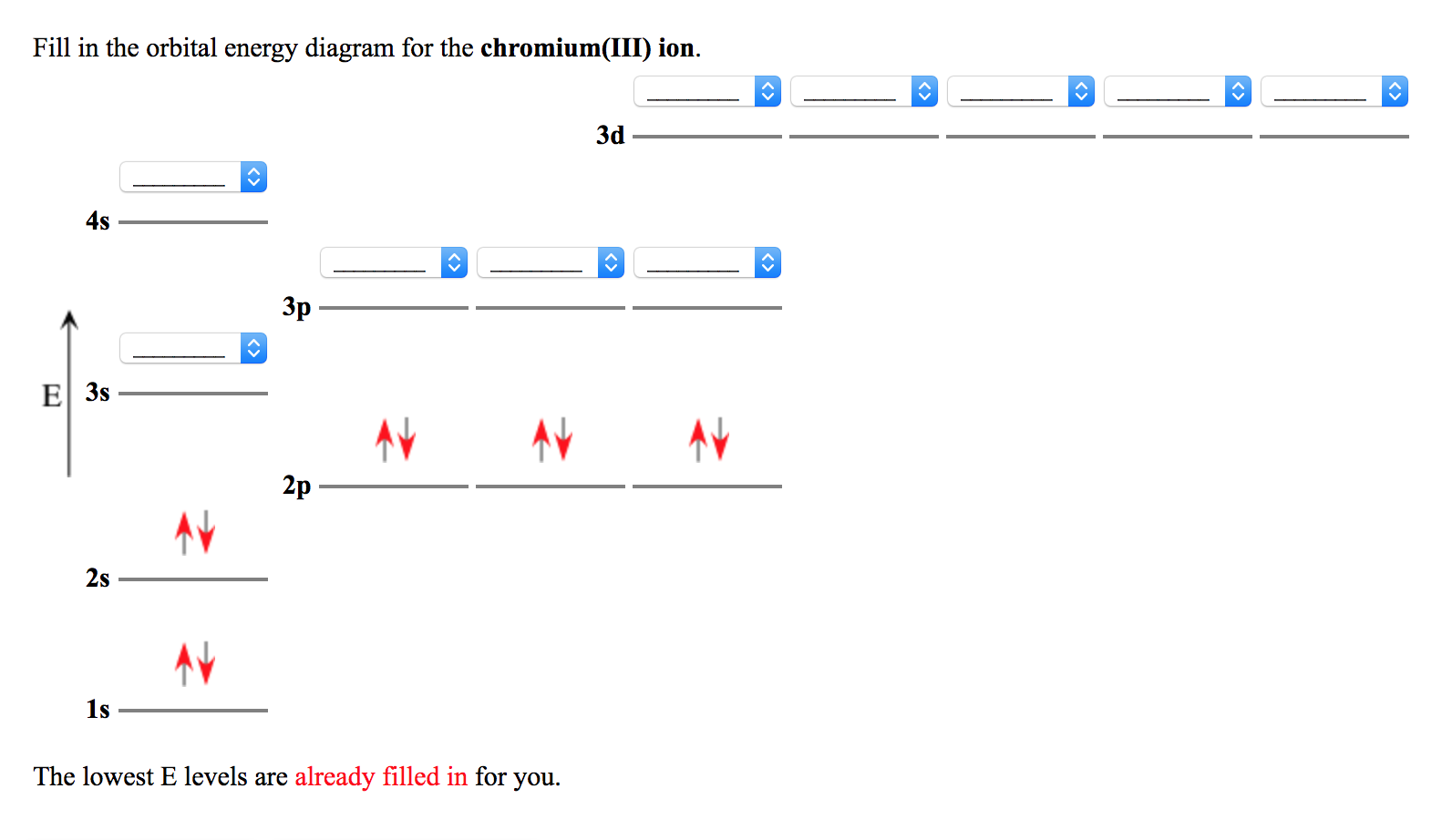











0 Response to "38 orbital diagram for cr3+"
Post a Comment