42 lewis diagram for bf3
BF3 Lewis Structure, hybridization | UO Chemists This blog will basically give a complete piece of information about boron trifluoride BF3. This will also explain the BF3 lewis structure, its structural properties as well. Structural properties explanation is based on the lewis structure. In simple words, we can say that drawing lewis's structure is necessary to explain all the structural properties including BF3 Lewis Structure: How to Draw the Lewis ... - YouTube A step-by-step explanation of how to draw the BF3 Lewis Dot Structure (Boron trifluoride).For the BF3 structure use the periodic table to find the total numb...
Lewis Structure (CH3)2O-BF3 - YouTube Made with Explain Everything
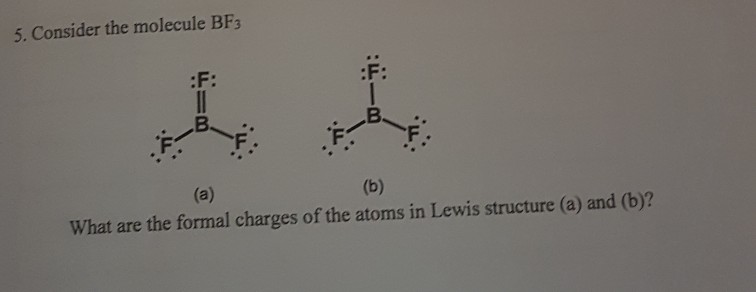
Lewis diagram for bf3
What is the Lewis structure of XeH4? - Give book Beside above, what is the Lewis structure for ClO? The chlorite ion, ClO 2 -, contains 19 (7 from the Cl and 6 from each of the two O atoms) +1 = 20 electrons.One electron is added because the entire molecule has a -1 charge. Hypochlorite ion Lewis structureThe hypochlorite ion, ClO −, contains 13 + 1 = 14 electrons. What is the Lewis structure of boron trifluoride ... Boron trifluoride (BF3) consists of a central boron atom with three single bonds to fluorine atoms. The boron atom is an exception to the octet rule, and generally only needs 6 electrons to be stable in a bonded molecule. The geometry of the BF3 molecule is called trigonal planar. Lewis Structure of BF3 - YouTube How to do the Lewis structure of BF3!Subscribe for more! If you have any questions or suggestions please include them in the comment below ;)Enjoy!
Lewis diagram for bf3. BF3 Lewis Structure: How to Draw the Dot Structure for BF3 ... Drawing the Lewis Structure for BF 3. Viewing Notes: The BF 3 Lewis structure is similar to BCl 3 and BBr 3 since Cl and Br are in Group 7 and have 7 valence electrons.; Boron (B) doesn't need 8 valence electrons to have an octet (Boron often only needs 6). If you're not sure you have the best Lewis structure for BF 3 you can calculate the formal charges. You'll find the B in BF 3 only has 6 ... Lewis Structure of Boron Trifluoride (BF3) Lewis structure of boron trifluoride (BF 3) is shown below and you can see each fluorine atom has made a single bond with boron atom. Boron atom is the center atom. Boron atom is the center atom. In this tutorial, we will learn how to draw the lewis structure of BF 3 with all theories. Lewis Structure for BF3 (Boron Trifluoride) - UMD Drawing the Lewis Structure for BF 3. Video: Drawing the Lewis Structure for BF 3. For the BF 3 Lewis structure, calculate the total number of valence electrons for the BF 3 molecule. There are a total of 24 valence electrons for the BF 3 Lewis structure. After determining how many valence electrons there are in BF 3, place them around the central atom to complete the octets. What is the Lewis structure of BF3? - Answers BF3 is a stronger Lewis acid as fluorine is more electronegative than iodine. How is the structure of boron trifluoride? The molecular formula is boron trifluoride is BF3.
BF3 Lewis structure, Molecular geometry, Hybridization ... BF3 Lewis structure, Molecular geometry, Hybridization, Polarity. Boron Trifluoride or trifluoroborane (BF3) is an inorganic compound with toxic properties. It is a colorless gas. It has a pungent smell and forms white fumes when exposed to moist air. Boron trifluoride is highly toxic when inhaled. It reacts with heat when exposed for a ... Solved The Lewis diagram for BF_3 is: The electron-pair ... The Lewis diagram for BF_3 is: The electron-pair geometry around the B atom in BF_3 is There are lone pair(s) around the central atom, so the geometry of BF_3 is The Lewis diagram for BeF_2 is: The electron-pair geometry around the Be atom in BeF_2 is There are lone pairs around the central atom. so the geometry of Be_F_2, is BF3 Lewis Structure (2022 UPDATED) Practical Guide In BF3, Boron is the least electronegative atom and will be used as the central atom. The Lewis Dot Structure will show you one Boron atom with three electrons in its last shell and three Fluorine atoms with seven electrons in its last shell. The computation will end with 24 total valence electrons, forming three B F bonds. Lewis Structures: BF3 - YouTube A Lewis structure for BF3.(Chem 1090 Lewis 7b)
BF3 Lewis Structure, Molecular Geometry, and Hybridization ... BF3 Lewis Structure, Molecular Geometry, and Hybridization. Boron Trifluoride (BF3) is an inorganic compound as it lacks a carbon atom or C-H bond in the molecule. Manufactured from the reaction of boron oxides and hydrogen fluoride, the chemical compound BF3 has a pungent smell and is colorless in nature. The compound behaves differently in ... BF3 Lewis Structure (Boron Trifluoride) - YouTube Hey Guys,Today we are going to look at the Lewis Structure of BrF3 which is a chemical formula for Bromine Trifluoride. The molecule comprises one Bromine at... BF3 Lewis Structure, Molecular Geometry, Hybridization ... BF3 Lewis Structure. To know about BF3 Lewis structure, we have to calculate the total number of valence electrons for the BF3 molecule. BF3 has a total of 24 valence electrons, which we have to set around the central atom. How to Draw a Lewis Structure for BF3 Boron Trifluoride ... How to Draw Lewis structure for BF3 Boron Trifluoride?Lewis Structure: ...
Answered: What is the Lewis Structure for BF3,… | bartleby What is the Lewis Structure for BF3, the steric number, the electron pair geometry, the molecular geometry, and how many double bonds does it have? check_circle Expert Answer. Want to see the step-by-step answer? See Answer. Check out a sample Q&A here. Want to see this answer and more?
Lewis Structure of BF3 - YouTube How to do the Lewis structure of BF3!Subscribe for more! If you have any questions or suggestions please include them in the comment below ;)Enjoy!
What is the Lewis structure of boron trifluoride ... Boron trifluoride (BF3) consists of a central boron atom with three single bonds to fluorine atoms. The boron atom is an exception to the octet rule, and generally only needs 6 electrons to be stable in a bonded molecule. The geometry of the BF3 molecule is called trigonal planar.
What is the Lewis structure of XeH4? - Give book Beside above, what is the Lewis structure for ClO? The chlorite ion, ClO 2 -, contains 19 (7 from the Cl and 6 from each of the two O atoms) +1 = 20 electrons.One electron is added because the entire molecule has a -1 charge. Hypochlorite ion Lewis structureThe hypochlorite ion, ClO −, contains 13 + 1 = 14 electrons.

0 Response to "42 lewis diagram for bf3"
Post a Comment