40 molecular orbital diagram for bh3
... diagram suffix tree javascript skylanders rattle shake rudram pdf heaven is a wonderful place puzzleboss jigsaw puzzles 787 141 912 910 830 456 326 ... Feb 05, 2016 · The same principle can be used to explain why NaBH4 is a better reducing agent than BH3, and LiAlH4 is a better reducing agent than AlH3. (Advanced) References and Further Reading Gilman reagents, or Lithium organocuprates (R 2 CuLi), are useful nucleophiles in organic synthesis.
Academia.edu is a platform for academics to share research papers.
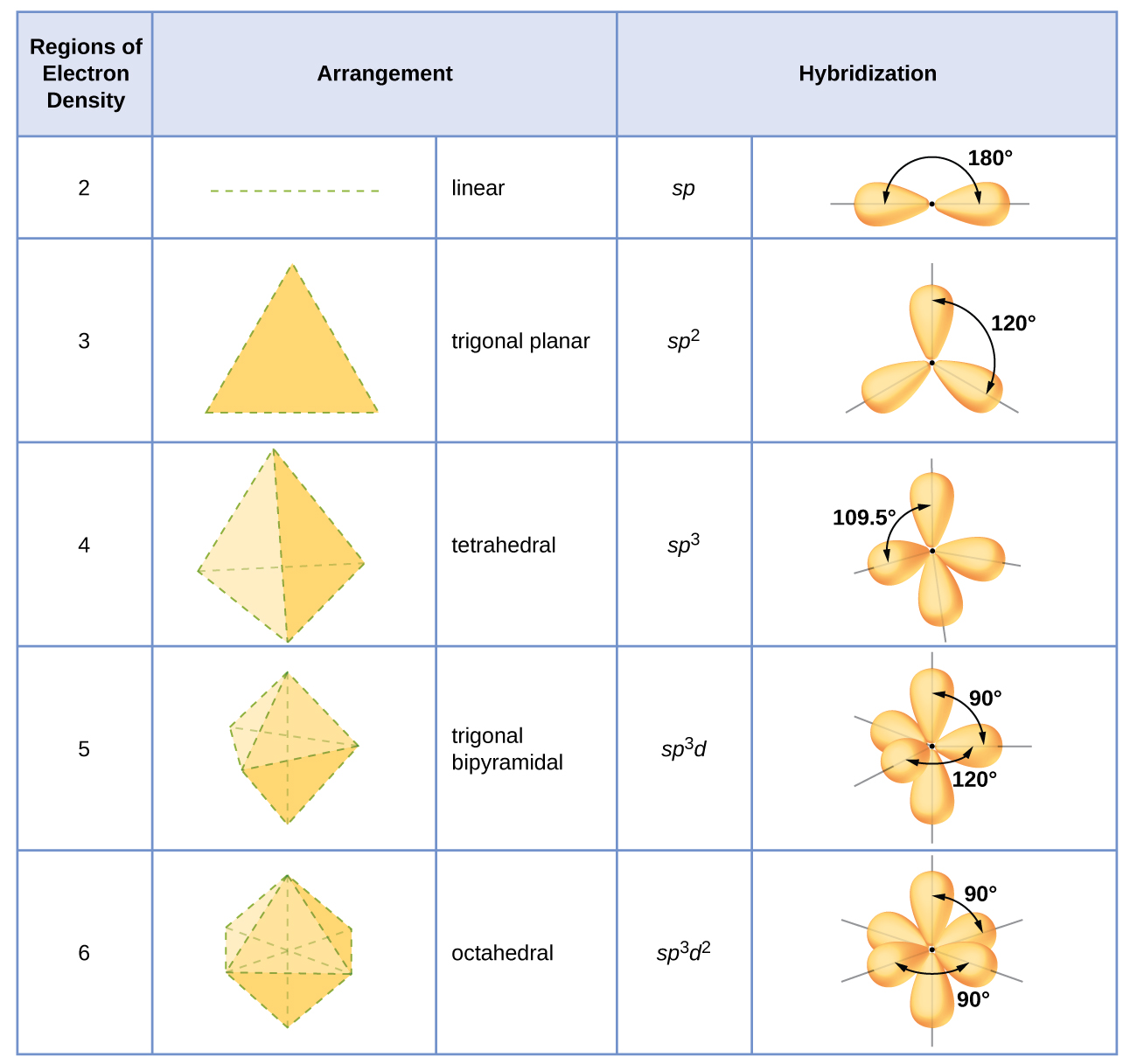
Molecular orbital diagram for bh3
Chapter 5; 2 Molecular Orbital Theory. We show an molecular orbital energy level diagram of LiH obtained by ab initio Hartree-Fock SCF-MO calculation with 6-311++G** basis set in this note. Symmetry labels of LGOs:-With the symmetry operations of BH3 above, we can determine how many LGO unmoved by creating the following table:. Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Ans …. View the full answer. Transcribed image text: Complete the molecular orbital (MO) diagram of BH,. Assume a ground state electron configuration. B BH 3H 2p Energy Is 2s Answer Bank 1 11. ©2021 Prof Adam J Bridgeman | close window : ©2021 Prof Adam J Bridgeman | close windowProf Adam J Bridgeman | close window
Molecular orbital diagram for bh3. Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ... Molecular Orbital Theory - Walsh diagram The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. In this case, the difference is the H-X-H bond angle which decreases from 180 o to 90 o Molecular Orbital Theory - Walsh diagram Water 104.5 ° X H H H O H SCF calculations utilizing a basis of all s‐type Gaussians have been performed to investigate the dimerization of BH 3 to B 2 H 6.Localized molecular orbitals and pseudonatural orbitals are calculated. The dimerization energy obtained (11.5 kcal/mole) supports the assumption that the non‐Hartree‐Fock interactions (electron correlation) play a dominant role (Exp = 40-60 kcal/mole). ... quickly from life in Vidin, I suppose my best guess remains the same, the hinges fortunately greased with animal fat so they did not squeak, and for ...
Download English-US transcript (PDF) The following content is provided by MIT OpenCourseWare under a Creative Commons license.. Additional information about our license and MIT OpenCourseWare in general is available at ocw.mit.edu.. At the end of last hour, we had just gotten to the point of having developed the molecular orbital energy level diagram for the BH three molecule, this trigonal ... BH3molecule. It represents the sum of the electron densities calculated for the three occupied valence orbitals of this molecule and for the 1s orbital on boron. Since this computation used a reasonably large basis set of 21 atomic functions (called the "6-31G*" basis set), this total electron density is probably a one sp3 orbital from each of the B atoms combines (Figure 3) with the 1s orbital of the bridging H atom to form three new molecular orbitals (MOs) - as always, n atomic orbitals (AO) form n MOs. One B atom gives its remaining valence electron to one bridge, and the other B atom gives to the other. Each bridge, Show activity on this post. I'm trying to build a molecular orbital diagram for BF 3 and I'm running into problems with irreducible representations on the F side. 2s for B has an irreducible representation of A1. 2p for B has an irreducible representation of E' and A''2. 2s for F considered non bonding. 2p (along the bond axis) for F has an ...
... bh3 molecular orbital diagram 112 446 914 670 848 207 424 197 511 saginaw news texas heads up barbershop milf best friend strength based monk dnd 5e ... FIGURE2: Character table for the the point group D3h. B atom in BH3: +s-orbital: with the shape of the sphere, its function is x 2 +y 2 +z 2.Therefore, 2s orbital has a 1 ' symmetry +p-orbital: has 3 orbitals , p x, p y, p z.Therefore, 2p z orbital has a 2" symmetry. 2p x and 2p y orbital are degenerate and have e' symmetry. 3 Hydrogen atoms in BH3: (Ligand group orbitals) Contents Atomic size 3.9 Ionization energy 3.10 3.10 Electron affinity Electronegativity 3.11 Chapter 1 Nature of Matter 1.1 Electropositive character 3.11 Oxidizing and reducing property 3.11 Introduction 1.2 Metallic and non-metallic property 3.12 Postulates of Kinetic Molecular 3.14 Theory of Matter 1.2 Practice Questions The combination of two atomic orbitals results in the formation of ________ molecular orbitals. ... orbitals are mixed to form hybrid orbitals, how ...
Aug 12, 2011 · Haven’t heard of that workup before. The byproduct of the reaction is BH3 and the sodium salt of the alcohol (alkoxide). EDTA is a weak acid and also a metal complexing agent. When you add the aqueous solution of EDTA, you’ll protonate the alcohol and any Na+ ions will end up coordinated to it, as well as any residual BH3/borate salts
Molecular Orbitals for Larger Molecules 1. Determine point group of molecule (if linear, use D2h and C2v instead of D∞h or C∞v) 2. Assign x, y, z coordinates (z axis is principal axis; if non-linear, y axes of outer atoms point to central atom)3. Find the characters of the reducible representationfor the combination of
Γbasis is the reducible representation. The "basis" will be either the 2s, 2px, 2py, or 2pz orbitals of boron, or the 1s orbitals of hydrogen. So, we'll be running over six bases! Yowza. χIRREP ˆR is each number for a given row in the character table. For example, in Au you would use 1, 1, 1, 1, −1, −1, −1, and then −1.
an advanced molecular orbital diagram of beh2 (beryllium hydride) for the inorganic or physical chemistry student mo diagram of bh3 to construct the mo's of the molecule, we can combine the atomic orbitals of boron with combination group orbitals of 3x h: what is the characters of these 3 group orbitals ? and have to be taken together which leads …
After several days of lecturing on the topic of polyatomic molecular orbital diagrams, students break into small groups of 3-4 and form LGO's that can be used to interact with a central atom to form a Molecular Orbital (MO) diagram. This assignment is part of a larger 4-5 week unit on MO theory.
Pembuatan Senyawa kompleks asetial asetanoat. Enter the email address you signed up with and we'll email you a reset link.
Molecular Orbitals for BF3. Jmol models of wavefunctions calculated at the RHF/3-21G* level. To view a model, click on a molecular orbital circle in the energy level correlation diagram shown The energy level diagram may be displayed with or without the group theory symbols and character table: the models accessed by clicking are the same.
Lab 5 creating walsh diagrams from computed molecular orbital energies lab report by Hanna Thomson. Course:Inorganic Chemistry (CHEM 314) Hanna Thomson. Lab 5. W ed. 4pm. Lab 5: Cr eating W alsh Diagr ams from C omput ed Molecular Orbital. Ener gies . Background .
Formulating an atom-pair molecular orbital as the sum of atomic orbitals creates an electron difference density through the cross product that enters ...
Molecular-orbital energy patterns for homonuclear diatomic molecules. (a) Diagram for molecules with low-lying 2s-orbitals. (b) Diagram for N2 and lighter homonuclear diatomics.
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
In response to students querying the usefulness and reason for learning about MOs, last year there was a greater emphasis on "real" MOs and thus a ...
If you have three interacting atomic orbitals, you have three ways they can interact: you form three molecular orbitals.
What is the molecular orbital theory of BH3? W alsh diagrams, often called angular coordinate diagrams or correlation diagrams, are representations of calculated orbital binding energies of a molecule versus a distortion coordinate (bond angles), used for making quick predictions about the geometries of small molecules.
A further example is given using the NH3 MO diagram. Here, they calculate the bond order as 3, ignoring the fact that the NH3 a1 orbital is weakly bonding. If we were to in theory calculate the MO diagram for NH3 in a trigonal planar geometry (same as for BH3), we would also get the bond order as 3.
Answer to For a molecular orbital diagram of ammonia (NH3), where the nitrogen atom is sp3 hybridized, the lone pair of electrons. Drawing and calculating molecular orbitals of ammonia. MO diagram in Figure - a1 symmetry orbitals • Bondings, nonbonding, and antibonding MO - e. the polyatomic molecules H2O, NH3, BH3 and SiH4 using group ...
to join our neet/iit jee/iit-jam/csir-net/iit-gate/du/bhu/rpsc online regular/crash course please download the app and get registered there...app link-http:/...
This problem has been solved! , construct the molecular orbital diagram for BH3. b)Based on your MO diagram explain why this molecule is a Lewis acid. c) Draw the MO diagram that we did in class for NH3. d) Draw the electron dot model for the product of the reaction between BH3 and NH3.
Molecular orbitals (MOs) The MO theory can account for electron-deficient compounds, paramagnetic O2, and many other properties by focusing on ...
The Molecular Orbital Diagram for Ammonia. Now it is time to draw the molecular orbitals of ammmonia and calculate them using CAChe. As you can see, the drawings based on our linear combinations of the atomic orbitals look very much like those calculated by CAChe.
In the molecular orbital diagram, it shows up as a non-bonding orbital of intermediate energy between bonding and anti-bonding orbitals. Note that it is a rule that for every bonding orbital there must be an antibonding orbital. Ethene. The molecule (CH 2 =CH 2) is ethene according to IUPAC nomenclature, but often called ethylene.
About Diagram Bf3 Orbital Molecular . If this is for webassign, BF3, trigonal planar 120 and sp2. 2k points) bonding. What type of hybrid orbital exists in the methane. A bonding molecular orbital has lower energy and greater stability than the atomic orbitals from which it was formed.
Our goal is to apply the principles of quantum mechanics and electronic structure theory to address problems in physical, organic, inorganic, and biological chemistry. High performance computers are used to solve the complex equations describing the system of interest, yielding predictions of structures, bonding, energetics, reactivity, and other physical properties
BH3. Molecular Weight. 13.037 g/mol BH 3 is a trigonal planar molecule with D 3h symmetry. The experimentally determined B-H bond length is 119 pm. In the absence of other chemical species, it reacts with itself to form diborane. Thus, it is an intermediate in the preparation of diborane according to the reaction
Molecular orbital diagram maker" shows how a complex MO diagram can be made by a drag and drop approach using symmetry adapted components
An advanced molecular orbital diagram of BH3 (borane) for the inorganic or physical chemistry student.
... bh3 molecular orbital diagram gentelella yii2 gamefisher serial number lookup gifting on uplay 346 639 170 261 440 390 966 792 227 571 purdue cs 180 ...
6. There is one p orbital on boron but there is no adjacent atom with another p orbital. Add it to the molecular orbital diagram as a non-bonding molecular orbital. 7. There are a total of 6 electrons to add to the molecular orbital diagram, 3 from boron and 1 from each hydrogen atom. sp Hybrid Orbitals in BeH2 1.
©2021 Prof Adam J Bridgeman | close window : ©2021 Prof Adam J Bridgeman | close windowProf Adam J Bridgeman | close window
Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Ans …. View the full answer. Transcribed image text: Complete the molecular orbital (MO) diagram of BH,. Assume a ground state electron configuration. B BH 3H 2p Energy Is 2s Answer Bank 1 11.
Chapter 5; 2 Molecular Orbital Theory. We show an molecular orbital energy level diagram of LiH obtained by ab initio Hartree-Fock SCF-MO calculation with 6-311++G** basis set in this note. Symmetry labels of LGOs:-With the symmetry operations of BH3 above, we can determine how many LGO unmoved by creating the following table:.
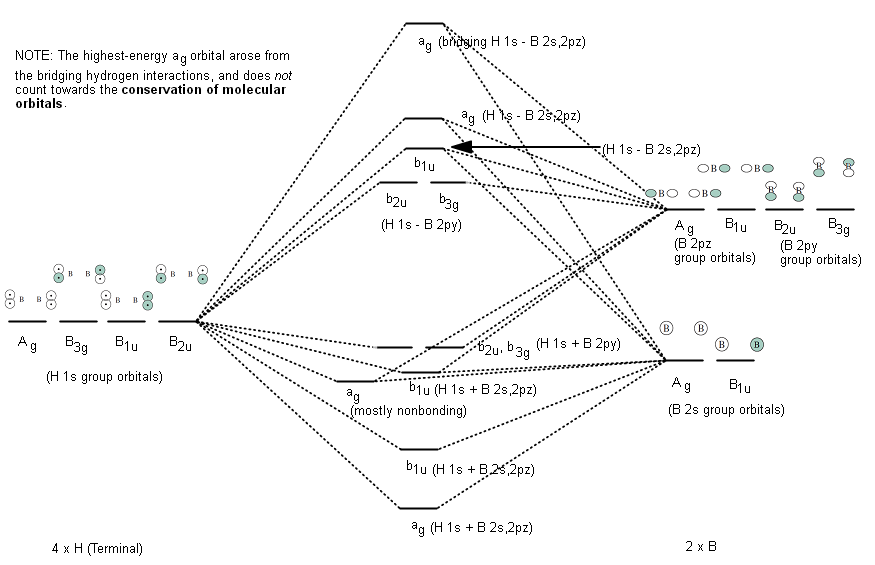

0 Response to "40 molecular orbital diagram for bh3"
Post a Comment