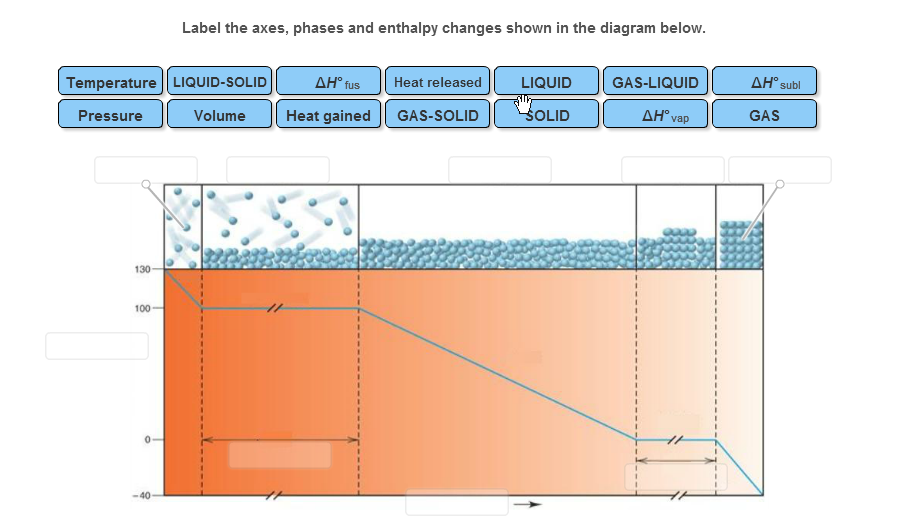
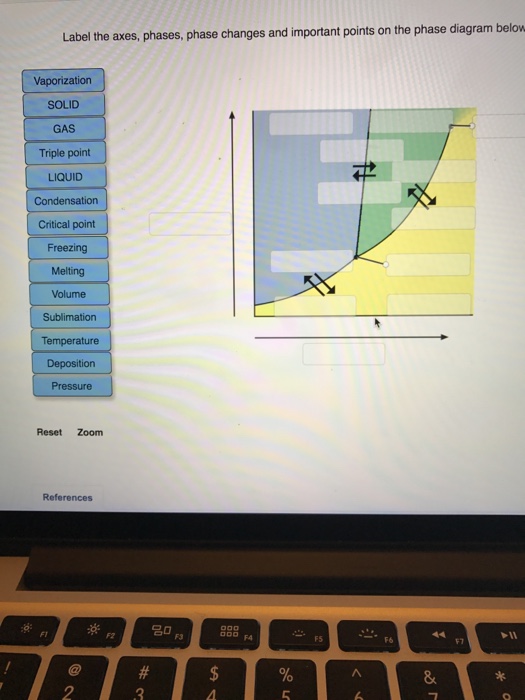
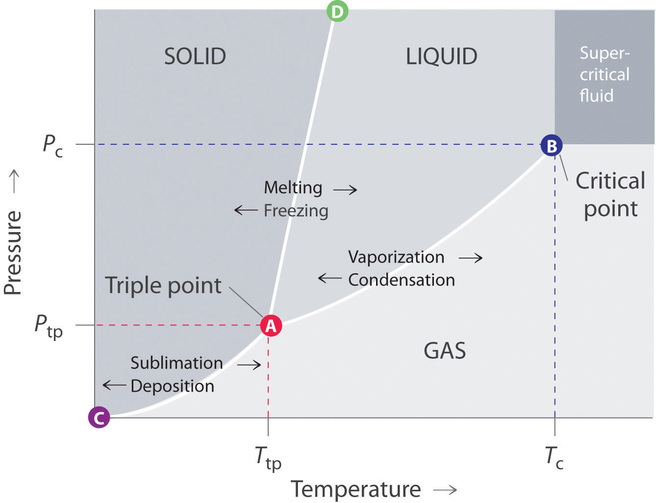
40 label the axes, phases, phase changes and important points on the phase diagram below.
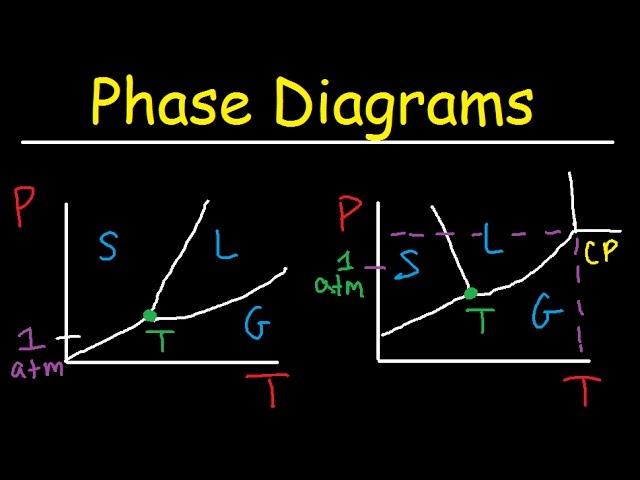
The simplest phase diagrams are pressure-temperature diagrams of a single simple substance, such as water.The axes correspond to the pressure and temperature.The phase diagram shows, in pressure-temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas.. The curves on the phase diagram show the points where the free energy (and other ... Label the axes, phases, phase changes and important points on the phase diagram below. Vaporization GAS Triple point SOLID 칸 Temperature f Sublimation Freezing Pressure KI Volume Condensation Deposition Melting Critical point LIQUID ; Question: Label the axes, phases, phase changes and important points on the phase diagram below. Vaporization ...
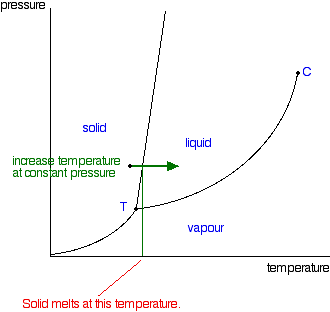
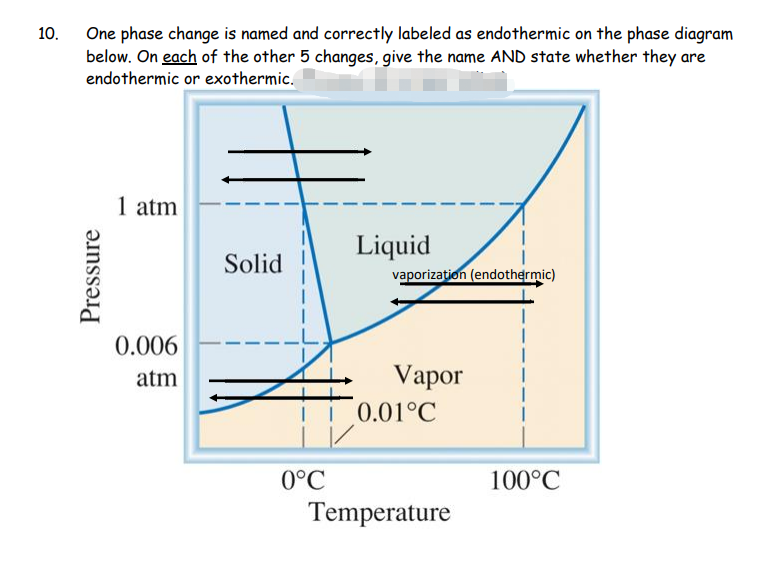
Label this heating curve with the phase or phases present. Decreasing temperature at constant pressure shifts the phases totally to solid see the red arrow labelled a in the phase diagram below. It is at those plateaus that a phase change occurs. Heating curves show how the temperature changes as a substance is heated up.

Label the axes, phases, phase changes and important points on the phase diagram below.
•The phase diagram displays a simple binary system composed of two components, A and B, which has a eutectic point. •The phase diagram plots relative concentrations of A and B along the X-axis, and temperature along the Y-axis. The eutectic point is the point where the liquid phase Chemistry. Chemistry questions and answers. Label the axes, phases, phase changes and important points on the phase diagram below. Sublimation SOLID Triple point Volume Temperature Melting Vaporization Deposition Condensation LIQUID GAS Pressure Freezing Critical point. Label the axes, phases, phase changes and important points on the phase diagram below Vaporization SOLID GAS Triple point LIQUID Condensation Critical point Freezing Melting Volume Sublimation Temperature Deposition Pressure Reset Zoom References.
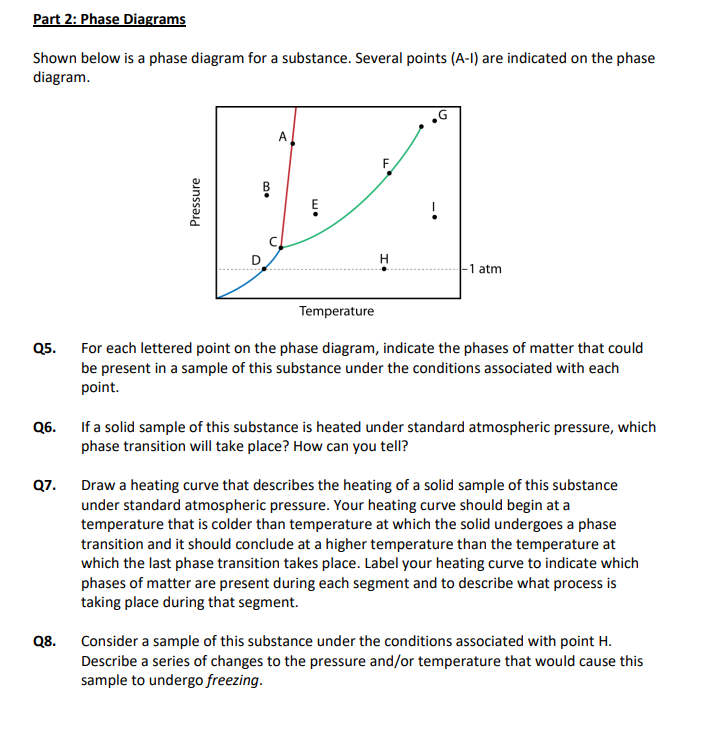
Label the axes, phases, phase changes and important points on the phase diagram below.. Q. Label the axes, phases, phase changes and important points on the phase diagram below. Q. How many grams of ice at -13.9 ∘C can be completely converted to liquid at 15.9 ∘C if the available heat for this process is 5.90×103 kJ ? the last figure with a few points labeled, and compare it to the phase diagram, also redrawn with important points labeled. Let's say we start out with a liquid of composition X. O B. and cool it to T. o. The gibbs free energy of the liquid would be given by point (1) on the g(X. B) diagram. The The molar heat of _______, symbol ∆Hfus, is the energy required to melt 1 mole of a solid. fusion. 23. The triple point is represented on a phase diagram to show when all three _________of a substance are at__________. Phases, equilibrium. 24. Which of the following statements are true concerning the vapor pressure of liquids and solids ... Chapter 9 - 10 Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram
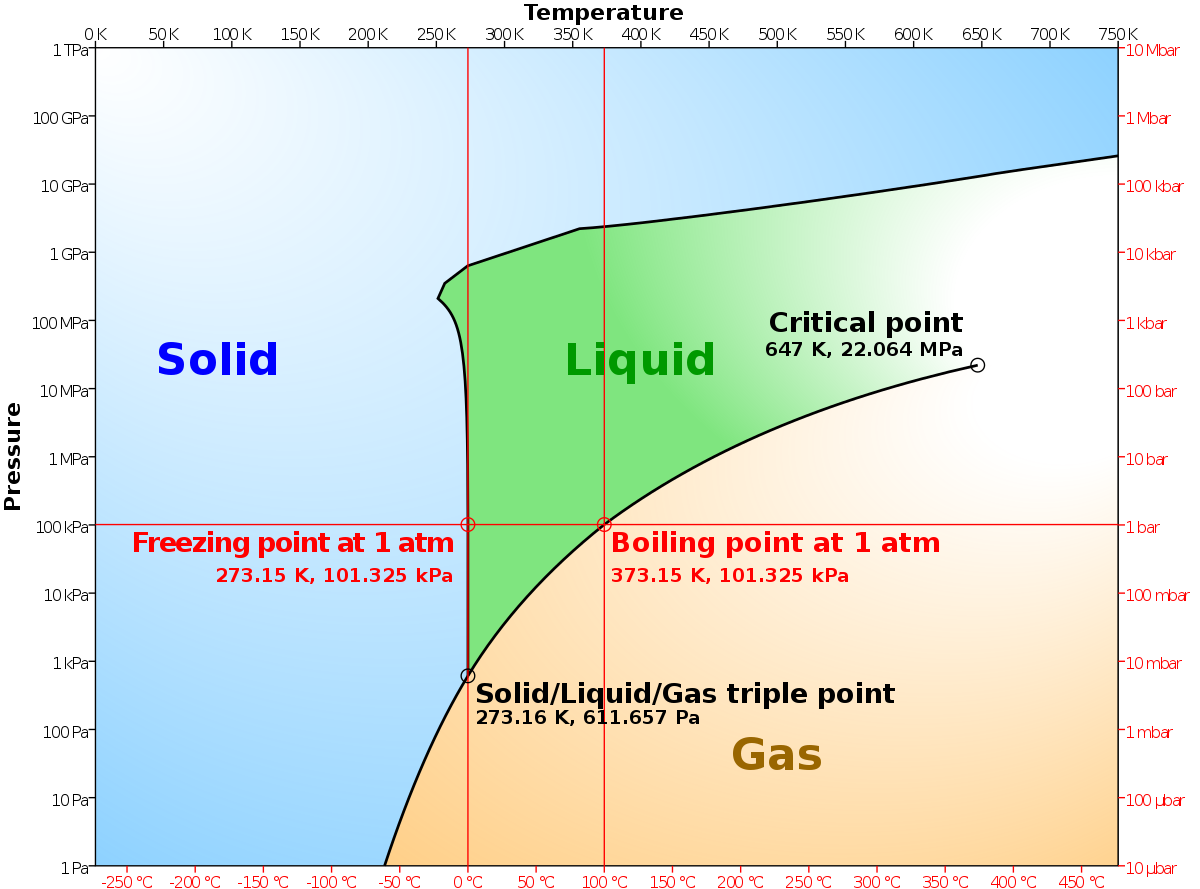
Draw the phase diagram for H2O. Label the phases, axes and two most important points. What is the significance of the shape of the line separating solid and liquid in question 1? Plot three isotherms for a real gas: above, at, and below the critical temperature (label). (b) Graphite is the most stable phase of carbon at normal conditions. On the phase diagram, label the graphite phase. (c) If graphite at normal conditions is heated to 2500 K while the pressure is increased to 10 10 Pa, it is converted into diamond. Label the diamond phase. (d) Circle each triple point on the phase diagram. Q. Label the axes, phases, phase changes and important points on the phase diagram below. Q. How many grams of ice at -13.9 ∘C can be completely converted to liquid at 15.9 ∘C if the available heat for this process is 5.90×103 kJ ? Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. A typical phase diagram has pressure on the y-axis and temperature on the x-axis. As we cross the lines or curves on the phase diagram, a phase change occurs. In addition, two states of the substance coexist ...
Label the axes, phases, phase changes and important points on the phase diagram below Vaporization SOLID GAS Triple point LIQUID Condensation Critical point Freezing Melting Volume Sublimation Temperature Deposition Pressure Reset Zoom References The Iron carbon equilibrium diagram (also called the iron carbon phase diagram) is a graphic representation of the respective microstructure states of the alloy iron - carbon (Fe-C) depending on temperature and carbon content. The iron carbon phase diagram is commonly used to fully understand the various phases of steel and cast iron. Comments on: Label the axes. phases. phase changes and important points on the phase diagram below. The tilt of Earth's axis causes the seasons because Choose one:A. one hemisphere of Earth is closer to the Sun in summer.B. the days are longer in summer.C. the rays of light strike the ground more directly in summer.D. both a and b are correct.E. both b and c are correct. E. both b and c are correct. Label these important reference points.
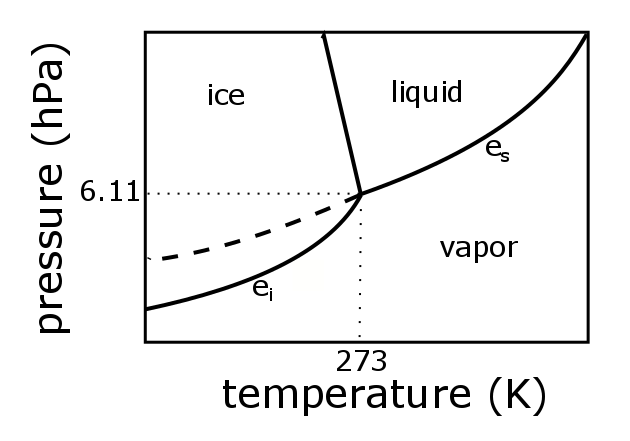
The phase diagram for carbon dioxide. The only thing special about this phase diagram is the position of the triple point which is well above atmospheric pressure. It is impossible to get any liquid carbon dioxide at pressures less than 5.11 atmospheres. That means that at 1 atmosphere pressure, carbon dioxide will sublime at a temperature of ...
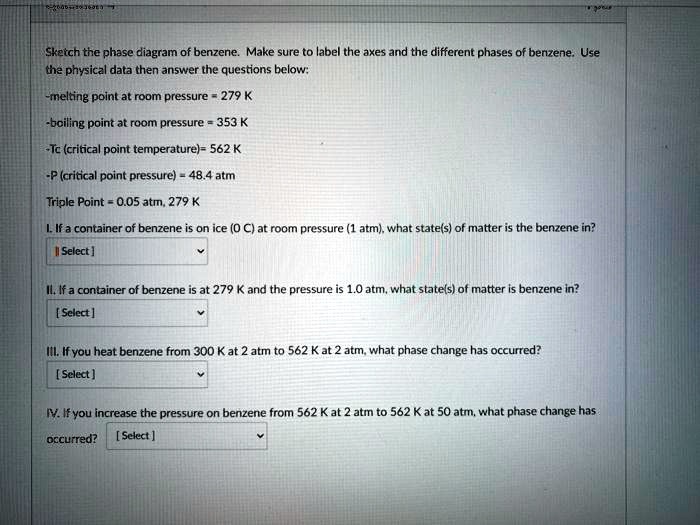
An example of a phase diagram for a normal substance, here benzene, is shown in the figure below. The diagram. Figure 13.1: Phase diagram of benzene showing P vs. T (left) and lnP vs. T (right). shows definite ranges of pressure P and temperature T for which the solid, liquid, and gas phases are stable. The line separating the solid and liquid ...
This problem asks that we determine the phase mass fractions for the alloys and temperatures in Problem 9.8. (a) From Problem 9.8a, ε and η phases are present for a 90 wt% Zn-10 wt% Cu alloy at 400 °C, as represented in the portion of the Cu-Zn phase diagram shown below (at point A).
Q. The pressure is increased on a sample of water at 0 °C from 0 mmHg to 800 mmHg. In order, what changes occur?
Problem: Label the axes, phases, phase changes and important points on the phase diagram below. FREE Expert Solution A phase diagram shows the transition of matter between solid, liquid, and gas phases as temperature and pressure changes. In a phase diagram, the vertical axis would be the pressure and the horizontal axis the temperature.
Label the axes, phases, phase changes and important points on the phase diagram below Vaporization SOLID GAS Triple point LIQUID Condensation Critical point Freezing Melting Volume Sublimation Temperature Deposition Pressure Reset Zoom References.
Chemistry. Chemistry questions and answers. Label the axes, phases, phase changes and important points on the phase diagram below. Sublimation SOLID Triple point Volume Temperature Melting Vaporization Deposition Condensation LIQUID GAS Pressure Freezing Critical point.
•The phase diagram displays a simple binary system composed of two components, A and B, which has a eutectic point. •The phase diagram plots relative concentrations of A and B along the X-axis, and temperature along the Y-axis. The eutectic point is the point where the liquid phase









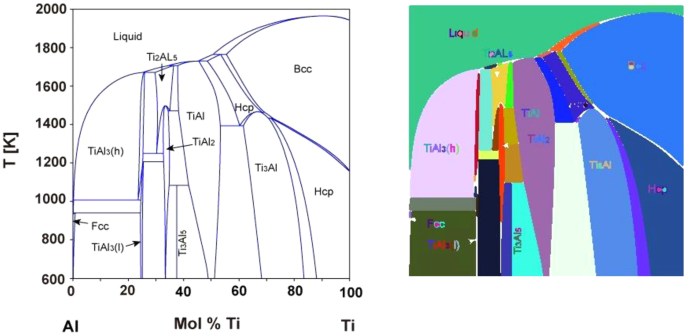
![Iron-Carbon Phase Diagram Explained [with Graphs]](https://fractory.com/wp-content/uploads/2020/03/Phase-diagram-of-steel-and-cast-iron.jpg)






![Iron-Carbon Phase Diagram Explained [with Graphs]](https://fractory.com/wp-content/uploads/2020/03/Iron-carbon-phase-diagram-explained.jpg)




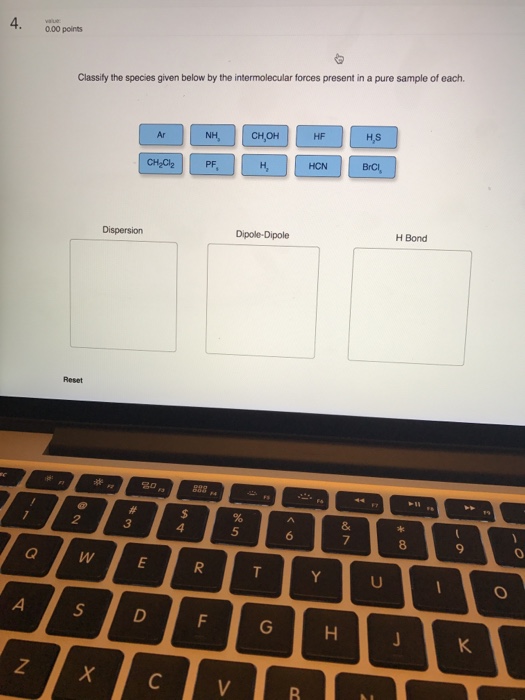

0 Response to "40 label the axes, phases, phase changes and important points on the phase diagram below."
Post a Comment