41 iron carbide phase diagram
The iron-carbon diagram (also called the iron-carbon phase or equilibrium diagram) is a graphic representation of the respective microstructure states depending on temperature (y axis) and carbon content (x axis). The melt essentially cools via the austenite to ferrite phases - i.e. from gamma to alpha mixed crystal. Concept of phase is explained with practical examples. With day to day life examples concept of unit cell is explained. After the completion of course students will grasp concept of Iron Iron Carbide Phase Diagram and will be able to sketch Iron Iron Carbide Phase diagram. Phase transformation temperatures are discussed in detail.
The Iron carbon equilibrium diagram (also called the iron carbon phase diagram) is a graphic representation of the respective microstructure states of the alloy iron - carbon (Fe-C) depending on temperature and carbon content. The iron carbon phase diagram is commonly used to fully understand the various phases of steel and cast iron.

Iron carbide phase diagram
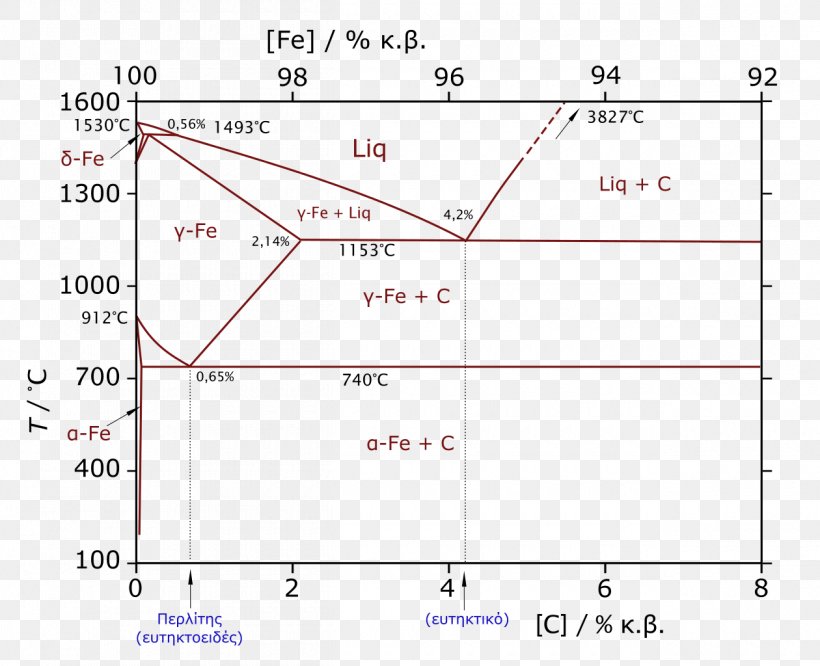
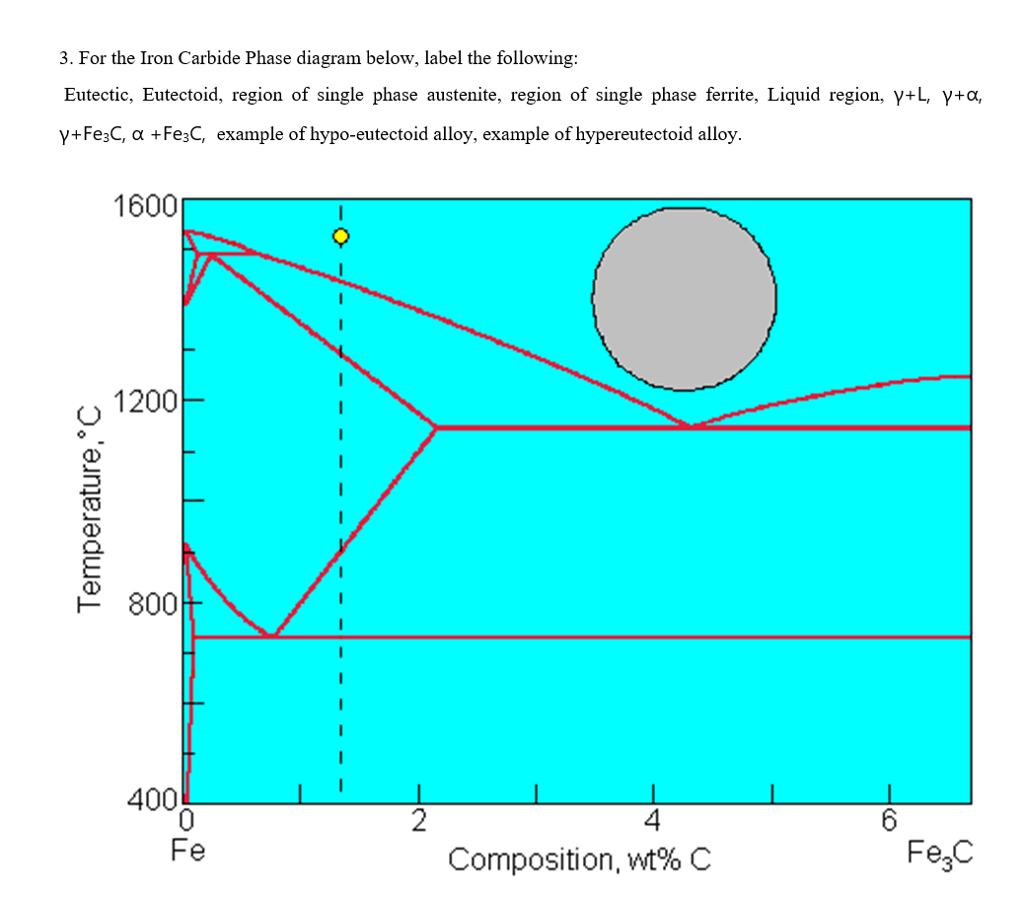
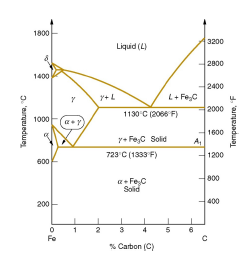
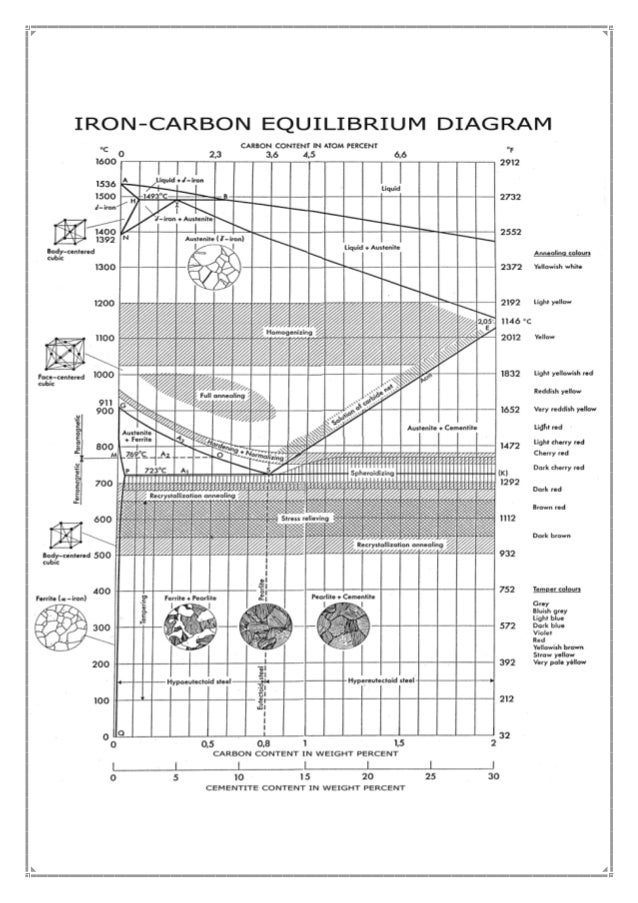
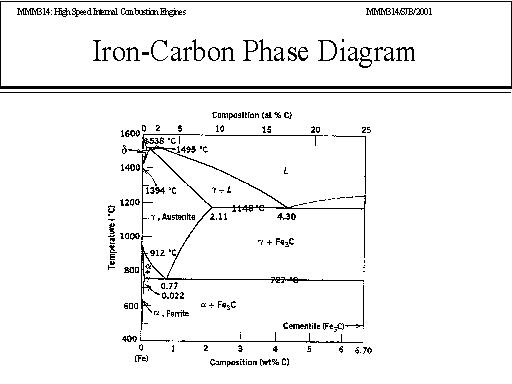
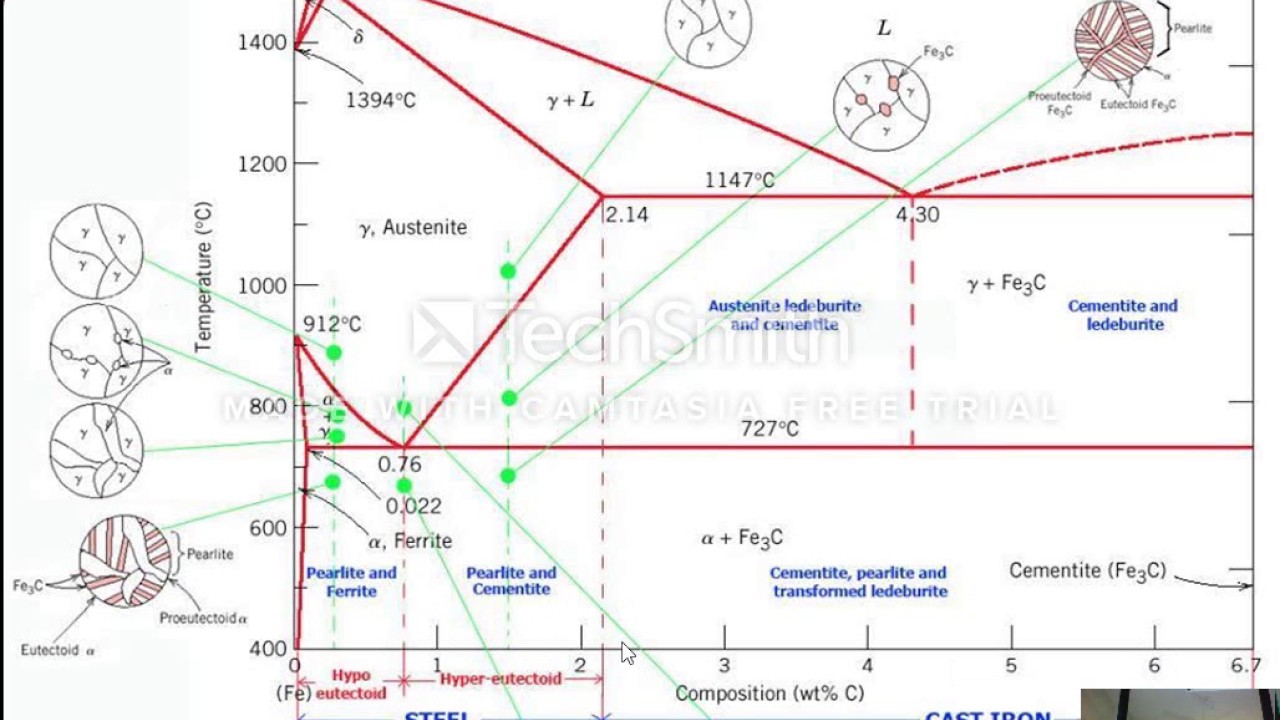
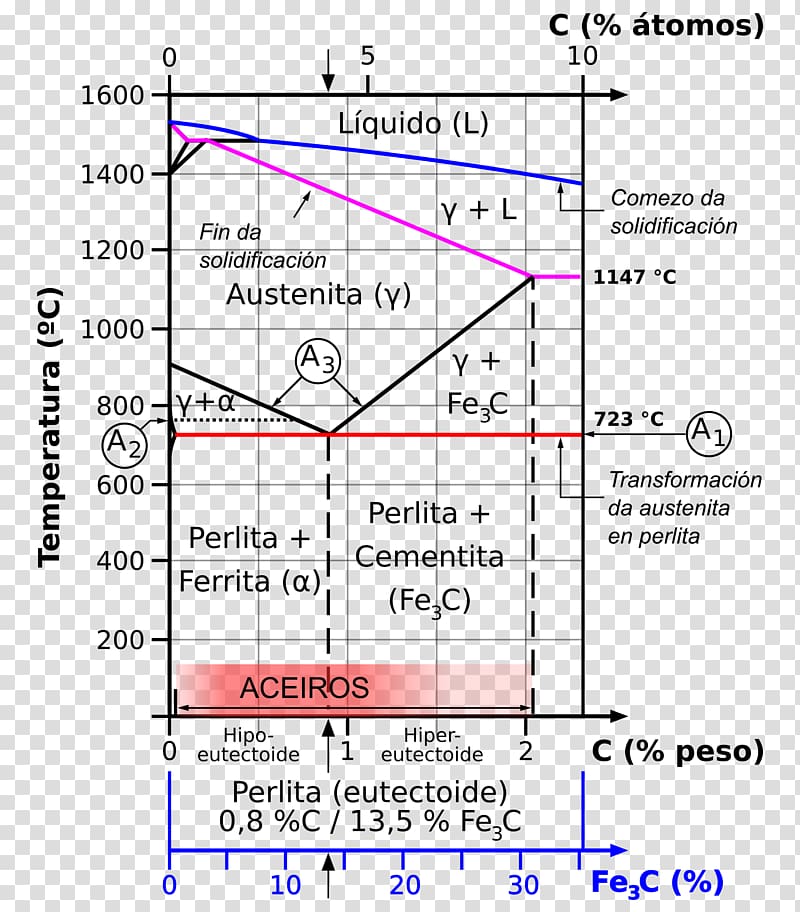
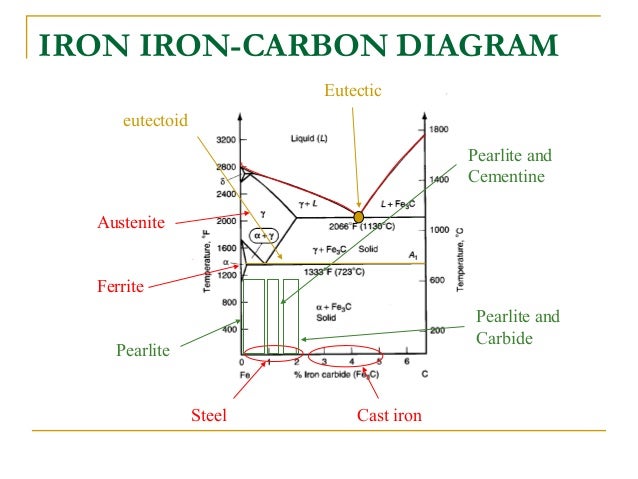
A very important phase change in the metastable Fe-C phase diagram occurs at 0.68 wt. % C. The transformation is eutectoid, and its product is called pearlite (ferrite + cementite) : gamma-iron (austenite) —> alpha-iron (ferrite) + Fe3C (cementite). Some important boundaries at single-phase fields have been given special names. These include : May 16, 2014 · THE IRON-IRON CARBIDE DIAGRAM The diagram shows three horizontal lines which indicate isothermal reactions (on cooling / heating): First horizontal line is at 1490°C, where peritectic reaction takes place: Liquid + d ↔ austenite Second horizontal line is at 1130°C, where eutectic reaction takes place: liquid ↔ austenite + cementite Third horizontal line is at 723°C, where eutectoid reaction takes place: austenite ↔ pearlite (mixture of ferrite & cementite) By: Muhd Hanzelah Zameer Khan Iron-carbon phase diagram Dr. Dmitri KopeliovichIron-carbon phase diagram describes the iron-carbon system of alloyscontaining up to 6.67% of carbon, discloses the phasescompositions and their transformations occurring with the alloys during their cooling or heating. Carboncontent 6.67% corresponds to the fixed composition of the iron carbide Fe3C.
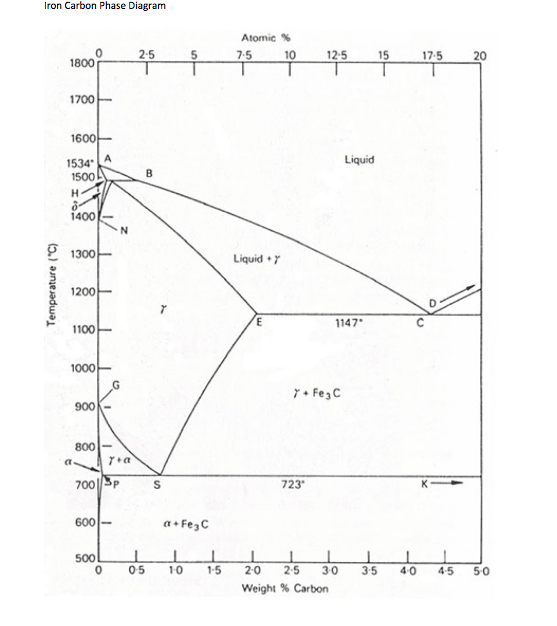
Iron carbide phase diagram. It has been stated that the iron-cementite phase diagram can be identified as the iron-ε-Fe 2 C carbide phase diagram in the concentration range of 0-9.7% C. General construction The phase composition depending on the temperature and the carbon content can be read off this dual diagram in which the stable system iron-graphite (dotted lines) and the meta-stable system iron-carbide (solid lines) are shown together, (Figure 1). Iron-Iron Carbide Phase Diagram | Material Engineering. The Iron-Iron carbide (Fe-Fe 3 C) is defined by five individual phases and four invariant reactions. Five phases are- α-ferrite (BCC) Fe-C solid solution, γ-austenite (FCC) Fe-C solid solution, δ -ferrite (BCC) Fe-C solid solution, Fe 3 C (iron carbide) or cementite – an inter- metallic compound and liquid Fe-C solution. So let's start with a phase diagram that contains maximal information: A 1: The upper limit of the ferrite / cementite phase field (horizontal line going through the eutectoid point). A2: The temperature where iron looses its magnetism (so-called Curie temperature ). Note that for pure iron this is still in the α -phase.
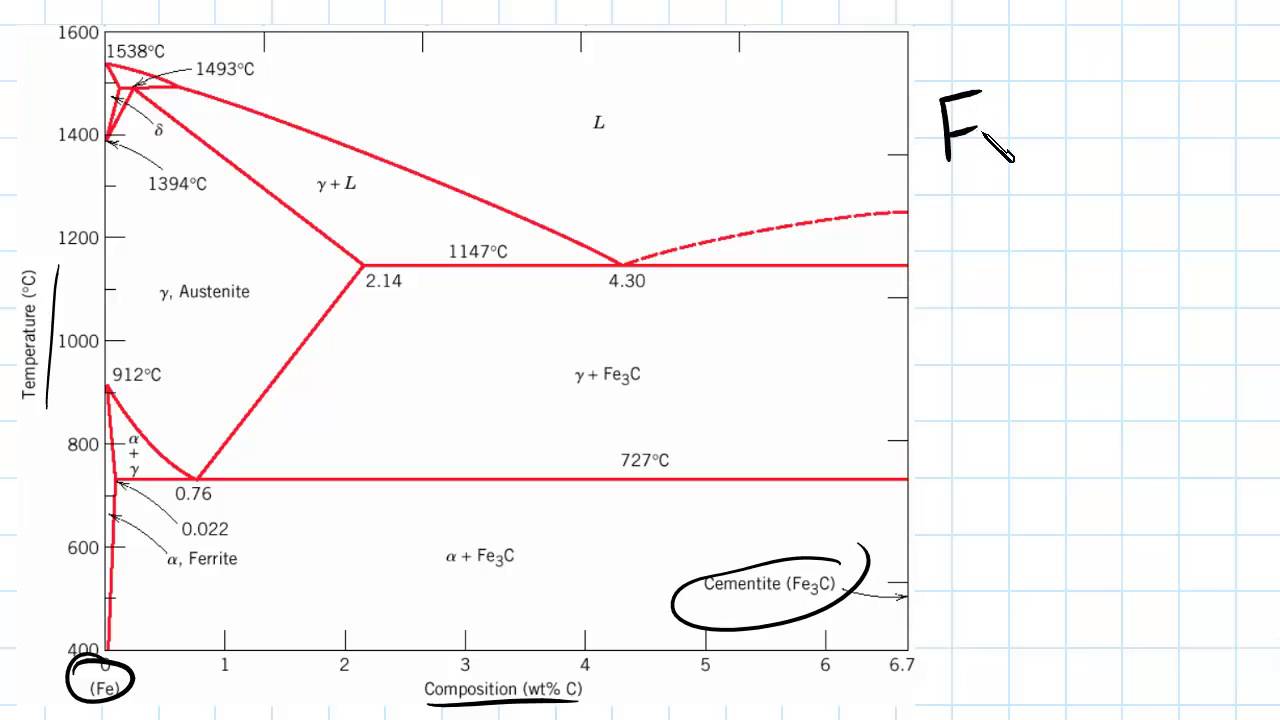
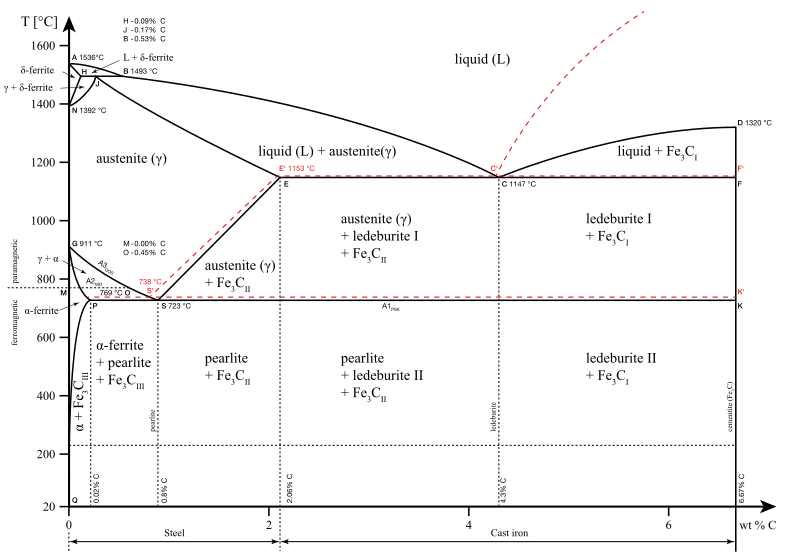
In the figure, there is the iron-iron carbide (Fe-Fe3C) phase diagram. The percentage of carbon present and the temperature define the phase of the iron carbon alloy and therefore its physical characteristics and mechanical properties. The percentage of carbon determines the type of the ferrous alloy: iron, steel or cast iron. The part of iron-carbon alloy system ... by weight is called iron-iron carbide equilibrium diagram. It may be noted that though it is called as equilibrium diagram, it is not a true equilibrium diagram, since equilibrium implies no change of phase with time.... called cast iron. When temperature of an alloy from this range reaches (1130 ºC), it contains primary austenite crystals and some amount of the liquid phase. The latter decomposes by eutectic mechanism to a fine mixture of austenite and cementite, called ledeburite. Phases in Fe–Fe. 3. C Phase Diagram. ¾α‐ferrite‐solid solution of C in BCC Fe. •Stable form of iron at room temperature. • Transforms to FCC g‐austenite at 912 °C ¾γ‐austenite‐solid solution of C in FCC Fe. • Transforms to BCC δ‐ferrite at 1395 °C •Is not stable below the eutectic temperature (727 °C) unless cooled rapidly. ¾δ‐ferritesolid solution of C in BCC Fe.
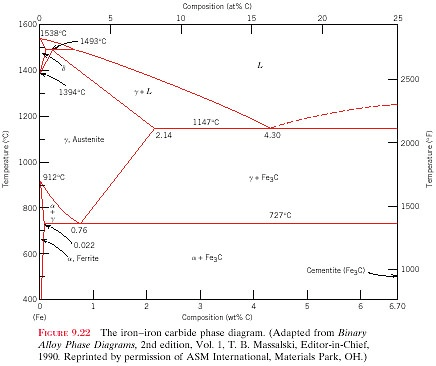
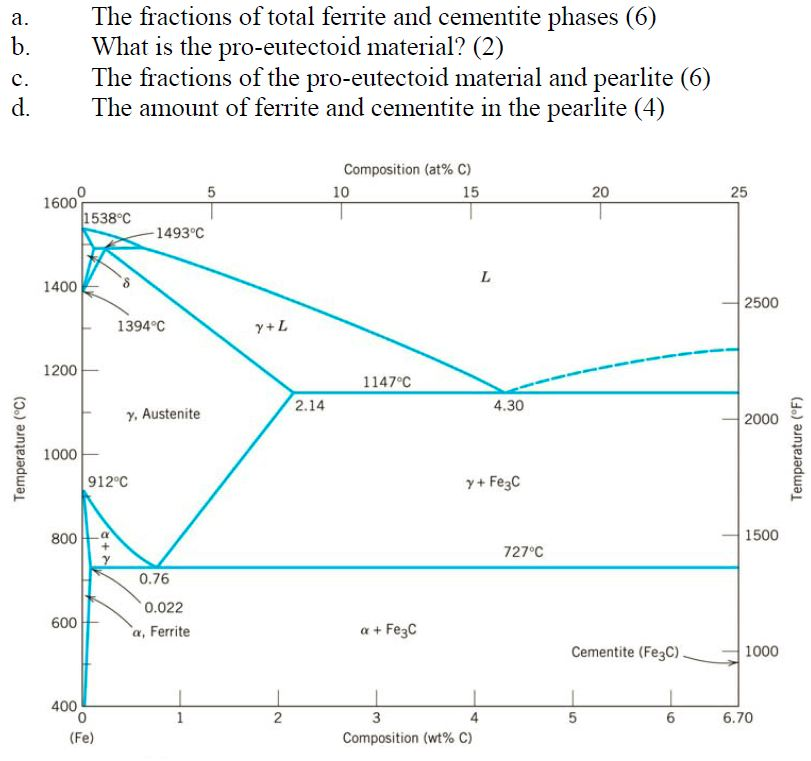
The iron -carbon phase diagram in Fig 2 actually shows two diagrams namely (i) the stable iron-graphite diagram (red lines), (ii) and the metastable Fe-Fe3C diagram. Cementite is metastable, and the true equilibrium is to be between iron and graphite (C). The iron-iron carbide phase diagram is shown in the Animated Figure 9.24. Question: Compute (a) the mass fractions of proeutectoid ferrite and (b) the mass fraction of pearlite that form in an iron-carbon alloy containing 0.30 wt% C. The iron-iron carbide phase diagram is shown in the Animated Figure 9.24. March 7, 2014 - Cementite Iron Carbide - an intermetallic compound Hard, brittle, white melts at 1837 C , density of 7.4 g/cc On the phase diagram, cementite corresponds to a vertical line at 6.7% C Engineers care only about compounds with less carbon Its presence in steels causes an increase in hardness ... IRON-IRON CARBIDE Phase Diagram 1. DEVELOPMENT OF MICROSTRUCTURE IN FE-C ALLOYS 1. SLOW COOLING OF EUTECTOID STEEL (0.76% C-STEEL) The figure shows the slow cooling of 0.76% C eutectoid steel. In the austenite range, this alloy consists of a uniform interstitial solid solution. Each grain of contains 0.76% C dissolved in the spaces of the FCC iron lattice structure. Nothing happens to ...
Principles of Physical Metallurgy by Prof. R.N. Ghosh,Department of Metallurgy and Material Science,IIT Kharagpur.For more details on NPTEL visit http://npt...
Access 130+ million publications and connect with 20+ million researchers. Join for free and gain visibility by uploading your research.
The iron-iron carbide (Fe-Fe3C) phase diagram Microstructures of iron α- ferrite austenite. 2 Interstitial sites of FCC Interstitial sites of BCC. 3 Microstructure in iron-carbon alloys REutectic--pearlite Hypoeutectoid alloys. 4 Hypereutectoid alloys Equilibrium diagrams having intermediate phases or compounds
Phase Diagrams (Iron-Iron Carbide Phase Diagram) Prof. Ratna Kumar Annabattula Department of Mechanical Engineering IIT MadrasSolid State Transofrmations, A...
Iron-Carbon Phase Diagram The iron-carbon phase diagram is widely used to understand the different phases of steel and cast iron. Both steel and cast iron are a mix of iron and carbon. Also, both alloys contain a small amount of trace elements.
The iron-iron carbide (Fe-Fe 3 C) phase diagram. The percentage of carbon present and the temperature define the phase of the iron carbon alloy and therefore its physical characteristics and mechanical properties. The percentage of carbon determines the type of the ferrous alloy: iron, steel or cast iron.
Iron-Carbon Phase Diagram with Detailed Explanation: If the percentage of the carbon is in the range of 0 to 2.11 % then it is called Steel and if the percentage of carbon is in the range of 2.11 to 6.67% then it is called Cast iron. As the carbon content increases, it produces more Iron-Carbide volume and that phase will exhibit high hardness.
October 8, 2016 - Iron carbon diagram presentation by Silver Star Enter... 116911 views · Iron Iron-carbide Equilibrium Phase... by Gulfam Hussain 4177 views
Iron carbide (Fe 3 C) is often labeled as the uncorroded portion of the steel. It is primarily associated with mild steels having a high carbon content and a ferritic-pearlitic microstructure. During corrosion of such steel, the ferrite phase dissolves and a porous iron carbide network is exposed (see Fig. 7.6).Given that iron carbide is an electronic conductor, this porous network serves as ...
Click on a date/time to view the file as it appeared at that time. ... The following pages on the English Wikipedia use this file (pages on other projects are not listed): ... View more global usage of this file. ... This file contains additional information, probably added from the digital ...
The Iron-Carbon Diagram: A map of the temperature at which different phase changes occur on very slow heating and cooling in relation to Carbon, is called Iron- Carbon Diagram. Iron- Carbon diagram shows - the type of alloys formed under very slow cooling, proper heat-treatment temperature and
Example: Phase Equilibria For a 99.6 wt% Fe-0.40 wt% C at a temperature just below the eutectoid, determine the following a) composition of Fe 3C and ferrite (α) b) the amount of carbide (cementite) in grams that forms per 100 g of steel c) the amount of pearlite and proeutectoid ferrite (α)
English: Iron-carbon phase diagram under atmospheric pressure. This diagram is limited by pure iron on the left and by iron carbide on the right. The mains phases are: * iron: ferrite, ferritic steel * iron: austenite, austenitic steel * iron carbide: cementite, Fe3C. We can see a eutectic and a eutectoid; these phases crystallise as a stacking ...
For a given temperature and carbon content, Iron will form in whatever phase requires the least amount of energy. The phase diagram diagram shows, in each 'segment', which phase or combination of phases requires the least amount of energy to form. Sometimes, the least-energy arrangement is a two-phase mixture.
Nptel is a joint initiative from IITs and IISc to offer online courses & certification. Learn for free, Pay a small fee for exam and get a certificate
Mechanical and industrial engineering offers a variety of professional opportunities in manufacturing, service, research and development, and public service enterprises.
Export articles to Mendeley · Get article recommendations from ACS based on references in your Mendeley library
Fundamentals of metals, Solidification, Phase transformations and phase diagrams ... , Electroplating, Adaptive tribological coatings, Metal joining technologies, Powder metallurgy, Steels and cast irons, Iron-carbon phase diagram, Effect of ... Magnesia ceramics, Zirconia ceramics, Aluminum titanate ceramics, Carbide ceramics ...
Accomplishing excellence in the technical education and act as repository and leader for disseminating state-of-the art knowledge and expertise in the field of materials technology and other relevant / emerging branches of engineering and technology concomitant with industrial growth of the ...
Important phases in Iron Iron Carbide Phase diagram has discussed in this video. Please subscribe to this our channel for more videos. #metallurgy #metallurg...
April 10, 2020 - A phase diagram is a graphical representations of the phases present in an alloy with a specific composition being held at a particular temperature.
Iron carbon phase diagram ferrite This solution has a melting point of 1538°C. Ferrite is the softest structure on the iron-iron carbide diagram. Ferrite acts magnetically at low temperatures, but its magnetic properties are dethatched once its temperature rises and above 786°C temperature it becomes non-magnetic.
The iron-carbon diagram (also called the iron-carbon phase or equilibrium diagram) is a graphic representation of the respective microstructure states depending on temperature (y axis) and carbon content (x axis). The melt essentially cools via the austenite to ferrite phases – i.e. from gamma to alpha mixed crystal.
Iron-carbon phase diagram Dr. Dmitri KopeliovichIron-carbon phase diagram describes the iron-carbon system of alloyscontaining up to 6.67% of carbon, discloses the phasescompositions and their transformations occurring with the alloys during their cooling or heating. Carboncontent 6.67% corresponds to the fixed composition of the iron carbide Fe3C.
May 16, 2014 · THE IRON-IRON CARBIDE DIAGRAM The diagram shows three horizontal lines which indicate isothermal reactions (on cooling / heating): First horizontal line is at 1490°C, where peritectic reaction takes place: Liquid + d ↔ austenite Second horizontal line is at 1130°C, where eutectic reaction takes place: liquid ↔ austenite + cementite Third horizontal line is at 723°C, where eutectoid reaction takes place: austenite ↔ pearlite (mixture of ferrite & cementite) By: Muhd Hanzelah Zameer Khan
A very important phase change in the metastable Fe-C phase diagram occurs at 0.68 wt. % C. The transformation is eutectoid, and its product is called pearlite (ferrite + cementite) : gamma-iron (austenite) —> alpha-iron (ferrite) + Fe3C (cementite). Some important boundaries at single-phase fields have been given special names. These include :

![Portion of Fe-C equilibrium phase diagram.[5] | Download ...](https://www.researchgate.net/profile/Tianyu-Yu-4/publication/320531737/figure/fig5/AS:668390926585883@1536368223982/Portion-of-Fe-C-equilibrium-phase-diagram5.jpg)


![The iron-carbon phase diagram [46]. | Download Scientific ...](https://www.researchgate.net/profile/Muna-Abbass/publication/293333803/figure/fig2/AS:669013386469398@1536516629671/Figure-2-11-The-iron-carbon-phase-diagram-46.png)

![iron-iron carbide Phase diagrams - [PPTX Powerpoint]](https://reader024.fdocuments.in/reader024/reader/2021022316/558cf0fed8b42aa4318b475a/r-35.jpg?t=1628883720)







![Iron-Carbon Diagram Explanation [PDF]](https://mechanicalenotes.com/wp-content/uploads/2018/10/Iron-Carbon-Phase-diagram-image.png)









0 Response to "41 iron carbide phase diagram"
Post a Comment