38 orbital diagram for oxygen
How to draw molecular Orbital Diagram of Oxygen molecule (O 2) ? Oxygen (O 2) molecule: Oxygen atom has electronic configuration 1s2, 2s2, 2p4 . Two p-atomic orbitals (one from each oxygen) atom combine to form two molecular orbitals, the bonding molecular orbital σ2px and antibonding molecular orbital σ*2px. Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels.
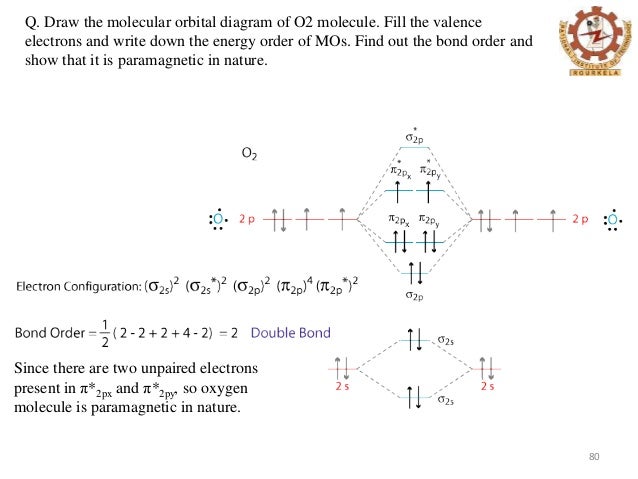
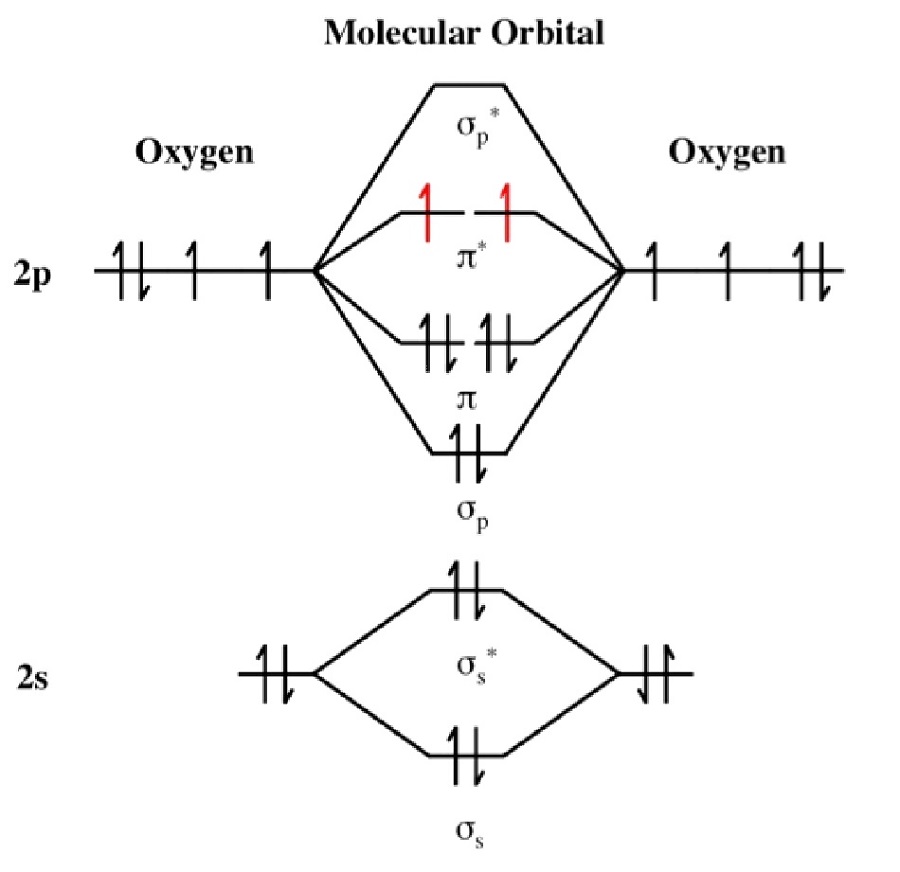
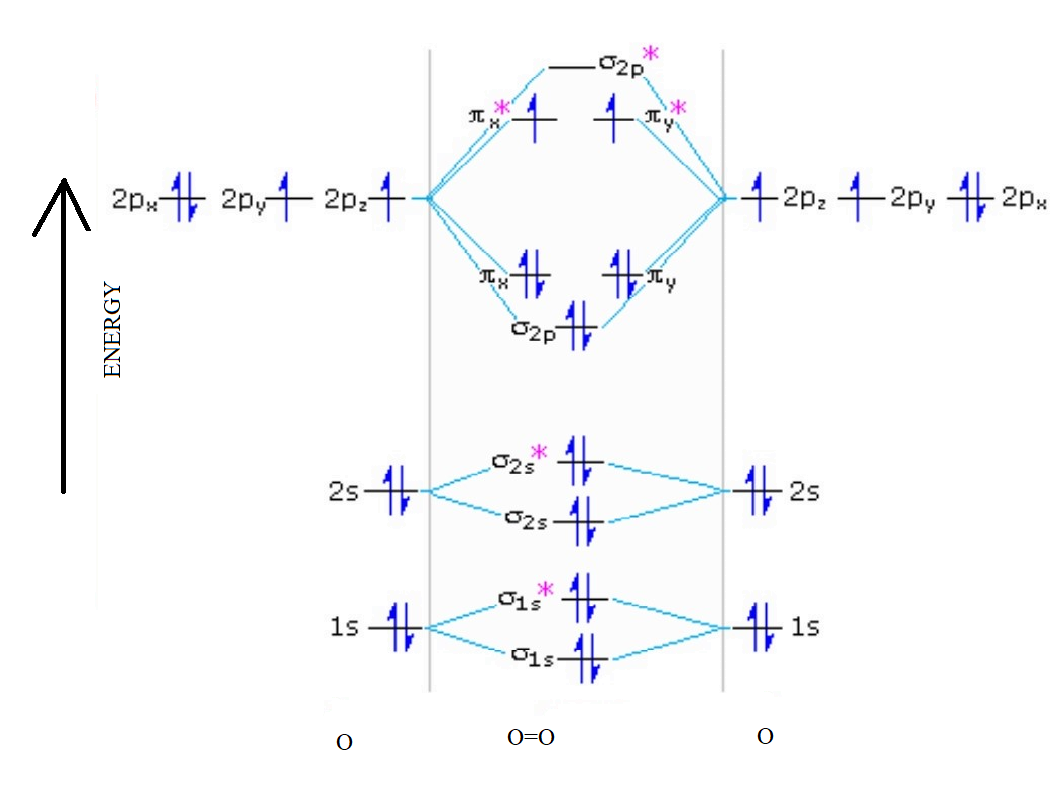
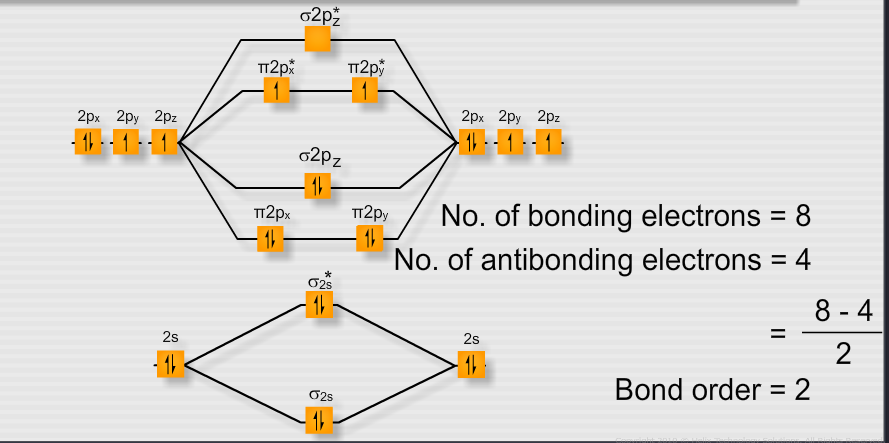
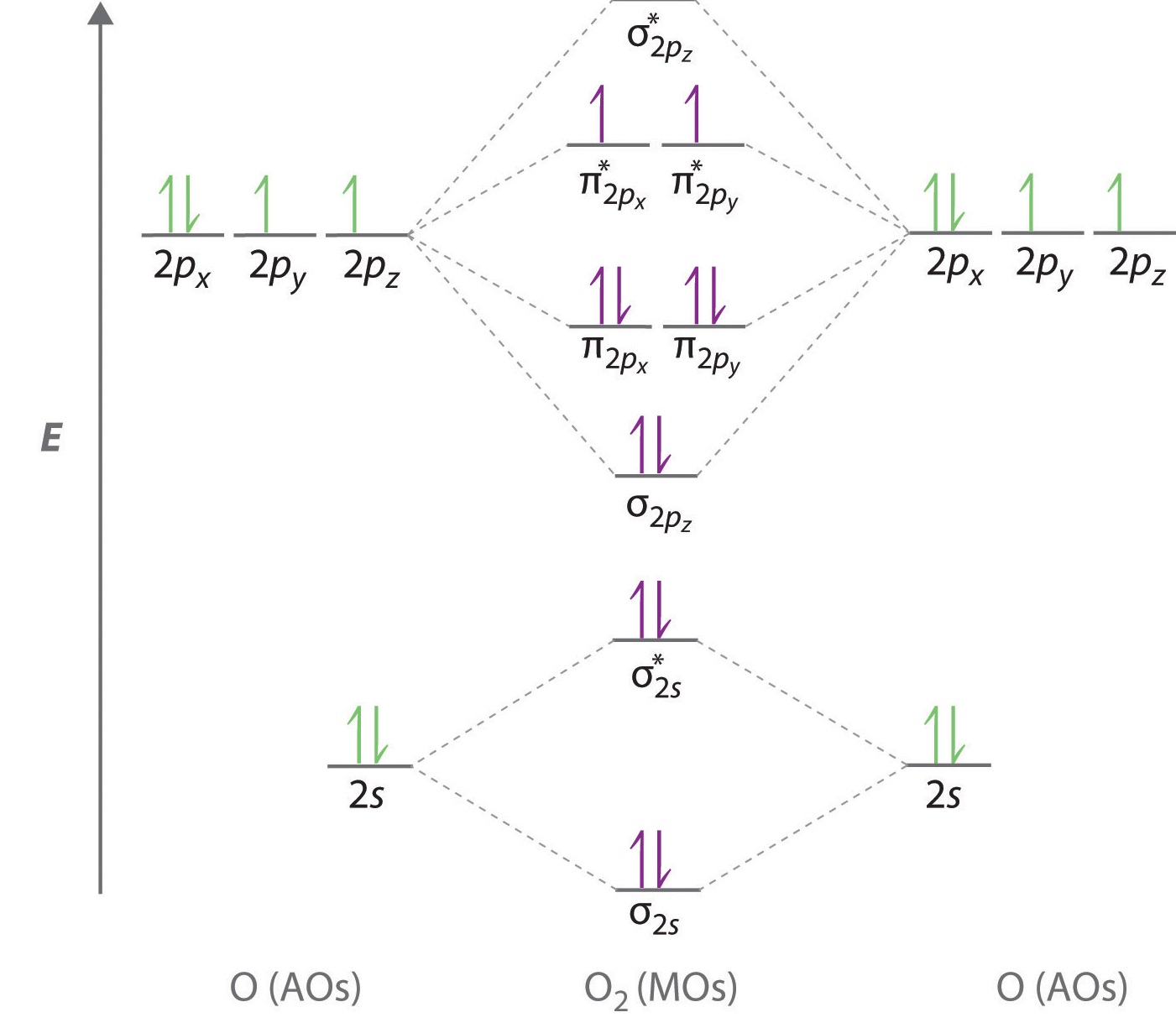
Electronic structure of oxygen atom is Leaving out the 4 electrons in the 1s orbitals of two oxygen atoms constituting the molecule (represented as KK), the molecular orbital energy diagram for remaining 12 electrons of oxygen as molecule is shown:(i) Electronic configuration:(ii) Bond order: Here Nb = 8; Na = 4The two oxygen atoms in a molecule of oxygen are united through two covalent bonds ...

Orbital diagram for oxygen
use because most oxygen is in the form of molecular oxygen (O2) at room temperature. Nevertheless, it is interesting to examine the energy diagram of the oxygen atom because similarities with the energy diagram of molecular oxygen can also be used to explain the reactivity of molecular oxygen. 1. Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ... Oxygen (O) electron configuration with full orbital diagram Oxygen (O) is the 8th element in the periodic table and the first element in group-16. The standard atomic mass of oxygen is 15.99903 and its symbol is 'O'. The period of oxygen is 2 and it is a p-block element.
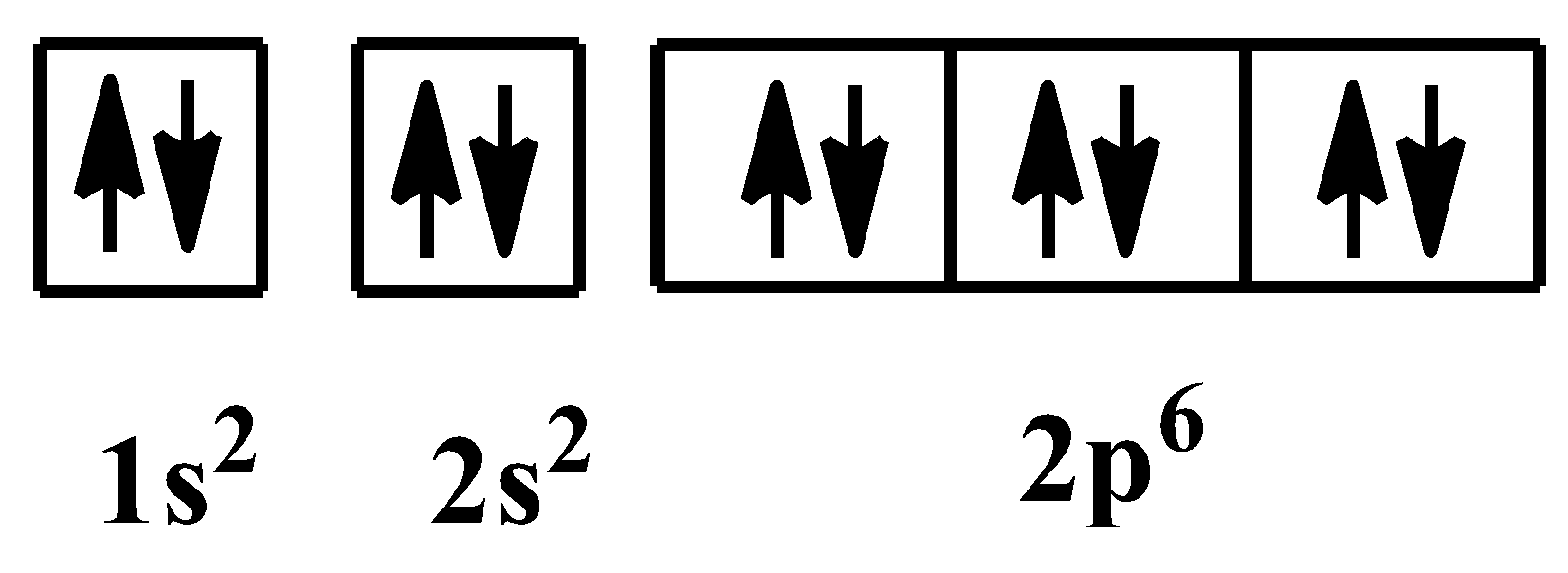
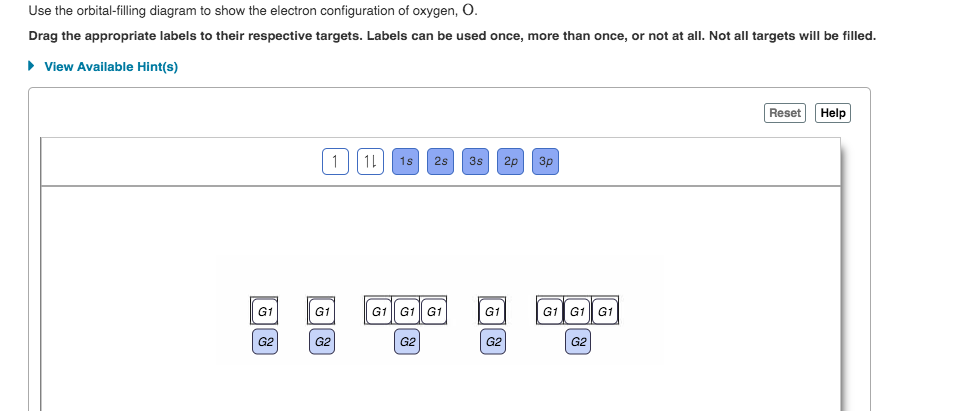
Orbital diagram for oxygen. In writing the electron configuration for oxygen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for O go in the 2s orbital. The remaining four electrons will go in the 2p orbital. Therefore the O electron configuration will be 1s22s22p4. Click to see full answer Click here to get an answer to your question ✍️ Identify the error(s) in the orbital notation diagram for oxygen in the in the image a . Electronic structure of oxygen atom is Leaving out the 4 electrons in the 1s orbitals of two oxygen atoms constituting the molecule (represented as KK), ... Rating: 4.4 · 740 votes · Free · Android · Educational Draw the valence shell molecular orbital diagram of the oxygen molecule and predict its magnetic nature. Answer. Verified. 118.2k+ views. Hint: The magnetic property of a molecule can be explained based on the molecular orbital theory. The molecule which does not contain the unpaired electron is known as the paramagnetic. The molecule which has ...
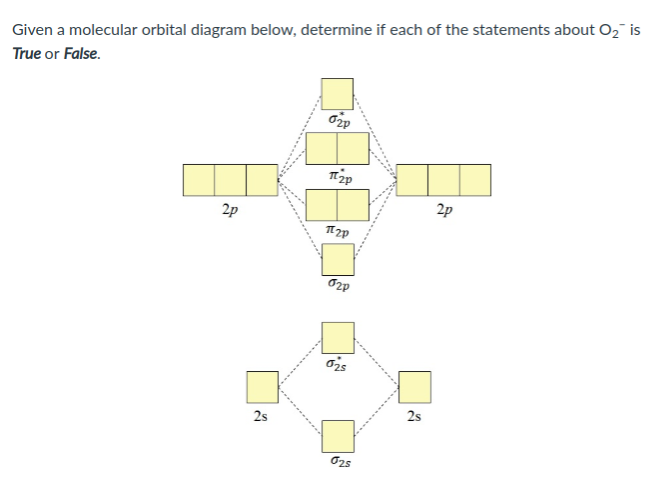
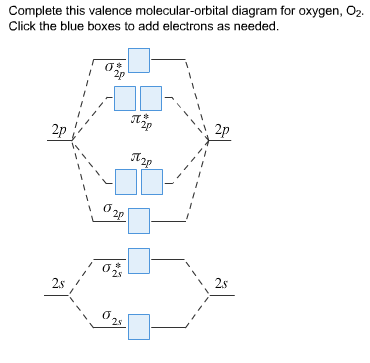
Molecular orbital diagrams of dioxygen molecule (left), superoxo state of oxygen (center), and peroxo state of oxygen (right). The molecular orbitals are constructed by the atomic orbitals of two ... Complete this valence molecular-orbital diagram for oxygen, O2. Click the blue boxes to add electrons as needed. Question: Complete this valence molecular-orbital diagram for oxygen, O2. Click the blue boxes to add electrons as needed. Consider the orbital diagram for oxygen (O) in Model 3. a. How many electrons are present in the orbital diagram? b. Based on its position in the periodic table, explain how you know that your answer to part a is the correct number of electrons for oxygen. III I II IV V 8 electrons The atomic number of oxygen is 8. Definition of atomic orbital diagram for oxygen: An orbital is the region of space around the nucleus within which the probability finding an electron of given energy is maximum. The diagram of this region gives the diagram of the orbital. The plot of angular wave function or square of angular wave functions give us the diagram of orbitals.
In writing the electron configuration for oxygen the first two electrons will move in the 1s orbital. Since 1s can most effective hang two electrons the subsequent 2 electrons for O go in the 2s orbital. The remaining four electrons will go in the 2p orbital. Therefore the O electron configuration can be 1s 2 2s 2 2p 4.. Hereof, what is the orbital diagram for phosphorus? Jan 21, 2021 — The electron configuration for O Ion is [He] 2s2 2p4. Full Electron Configuration For Oxygen. Oxygen's Atomic no. = 8 So the configuration is ... The only orbitals that are important in our discussion of molecular orbitals are those formed when valence-shell orbitals are combined. The molecular orbital diagram for an O 2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions between the 2s and 2p valence orbitals. Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Show Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 7.7.12. Each oxygen atom contributes six electrons, so the diagram appears as ...
Oxygen has four 2 p electrons. After each 2 p orbital has one electron in it, the fourth electron can be placed in the first 2 p orbital with a spin opposite ...
Molecular Orbital Diagram for Oxygen Gas (O2).Fill from the bottom up, with 12 electrons total.Bonding Order is 2, and it is Paramagnetic.sigma2s(2),sigma2s*...
One atom of oxygen has 8 electrons. Thus, two atoms will possess 16 electrons i.e. Oxygen molecules will have 16 electrons. The molecular orbital diagram of an Oxygen molecule is as - From the diagram, the electronic configuration of oxygen molecule can be written as -
When two oxygen atoms overlap, the sigma(2p) molecular orbital is LOWER in energy than the pi(2p) orbitals. This different from Nitrogen, where it's the othe...
The orbital diagram for a diatomic molecule is. To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels in the diagram) one at a time until you have used them up. They completely fill all the orbitals except the highest-energy antibonding sigma 2p orbital.
The orbital diagram is also one way of representing the electron configuration. Answer and Explanation: 1 Become a Study.com member to unlock this answer! Create your account View this answer The...
The bond length in the oxygen species can be explained by the positions of the electrons in molecular orbital theory. To obtain the molecular orbital energy-level diagram for (ce{O2}), we need to place 12 valence electrons (6 from each O atom) in the energy-level diagram shown in Figure (PageIndex{1}).
Click here👆to get an answer to your question ️ Draw molecular orbital diagram of O2 or N2 with magnetic behavior and bond order.
According to the MO diagram, oxygen has a bond order of 2. According to the MO diagram, oxygen has a bond order of 3. The Lewis structure for oxygen shown is accurately represented by the MO diagram below. Based on the completed MO diagram, it is clear that pi-type overlap is a weaker interaction than coaxial overlap.
The orbital diagram for a ground-state oxygen atom is 1s (up down) 2s (up down) 2p (up down, up, up) Which of the following is the electron configuration of an excited state of an oxygen Therefore, the electronic configuration of O In case of these elements, the order of energy levels of `sigma`2pxm, `pi` 2px and `pi`2py is reversed.
Sep 15, 2016 — The electron configuration for oxygen is: 1s^2 2s^2 2p^4 This video will walk you through the step of writing orbital diagram.1 answer · http://www.thestudentroom.co.uk/showthread.php?t=3933809&page=30 Explanation: The electron configuration for oxygen is: 1s22s22p4 This video will ...
From the molecular orbital diagram, we observe that oxygen has two unpaired electrons which is consist with the paramagnetic nature of oxygen. 6.6K views ·.4 answers · 8 votes: Four electrons in the lowest levels, sigma-1 and sigma*-2 (bonding and antibonding), two in ...
1 answerExplanation: The electron configuration for oxygen is: 1s22s22p4. expand. Was this answer helpful? upvote 0. downvote 0. Similar questions star-struck ...
Draw the molecular orbital diagram for oxygen molecule. Learning objective to draw, interpret and convert between Lewis (Kekule), condensed and bond line structures Note: The revision of the general chemistry in the Sections 1.3 - 1.6 is integrated into the learning goal above for the chemical organ ¢ Nica in the Sections 1.7 and 1.8.
Oxygen is the eighth element with a total of 8 electrons. In writing the electron configuration for oxygen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for O go in the 2s orbital. The remaining four electrons will go in the 2p orbital.
Either way, the Oxygen electron configuration will be 1s2 2s2 2p4 Once we have this information we can then place electrons in an orbital ...
Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 11.
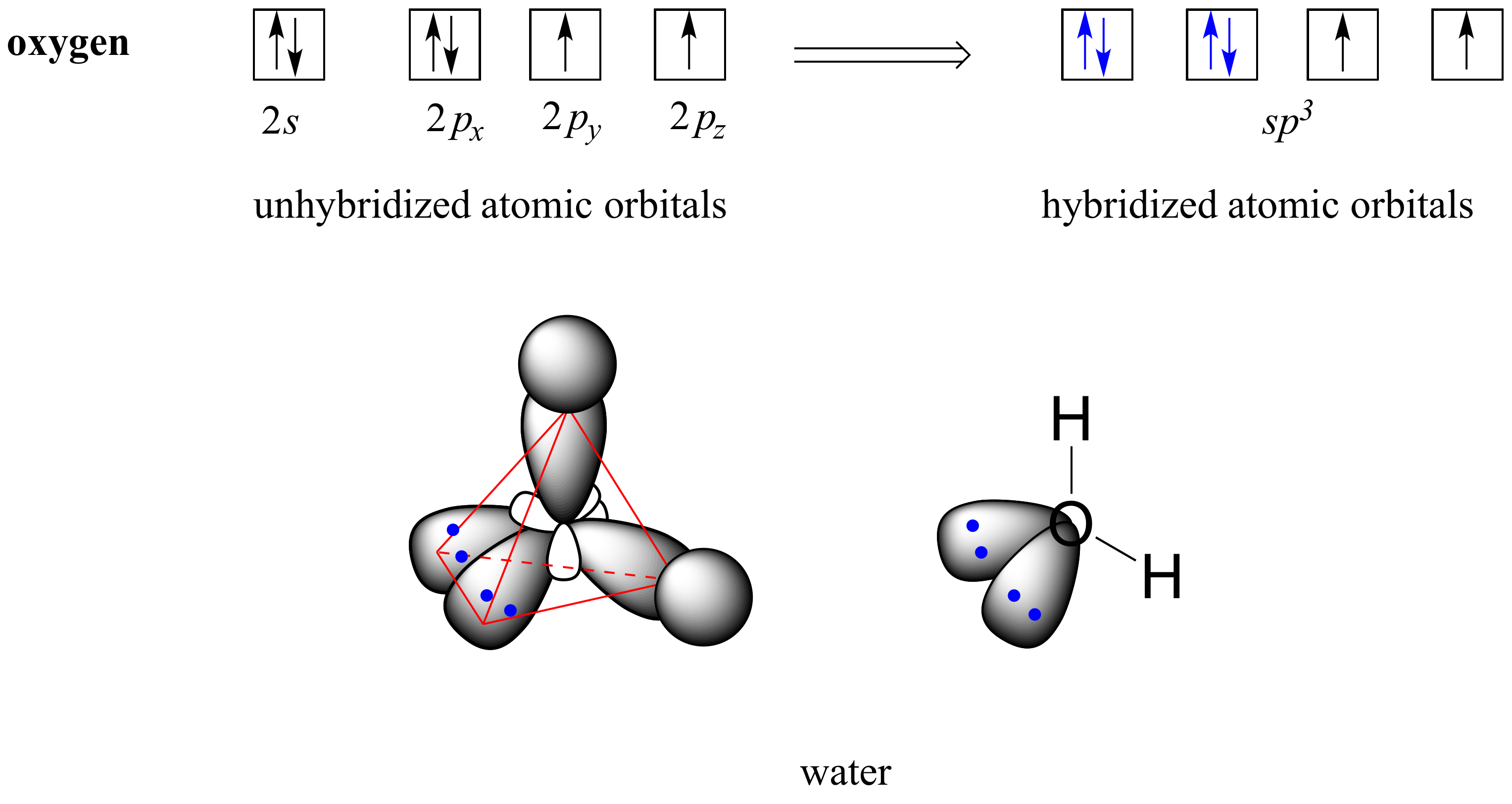
A. Fill in the atomic orbital diagram for oxygen. 2p Answer Bank 2px 2py 2pz Energy ไม้ | Question: Give the electronic configuration of the oxygen (O) atom. Show it both as an electron-populated orbital diagram and as a list of orbitals with superscripts indicating the number of electrons.
It occupies the 16th group and 2nd period of the periodic table. - The atomic number of oxygen atoms is 8 and the mass number of oxygen atoms is 16. - The ...1 answer · Top answer: Hint: Orbital diagram is the filling of the electrons into different orbitals according to the number of electrons present in an atom and an orbital consists ...
Oxygen (O) electron configuration with full orbital diagram Oxygen (O) is the 8th element in the periodic table and the first element in group-16. The standard atomic mass of oxygen is 15.99903 and its symbol is 'O'. The period of oxygen is 2 and it is a p-block element.
Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ...
use because most oxygen is in the form of molecular oxygen (O2) at room temperature. Nevertheless, it is interesting to examine the energy diagram of the oxygen atom because similarities with the energy diagram of molecular oxygen can also be used to explain the reactivity of molecular oxygen. 1.












![Molecular orbitals of triplet oxygen [19]. Molecular ...](https://www.researchgate.net/profile/Peter_Van_Puyvelde2/publication/318010903/figure/download/fig14/AS:660984981106688@1534602508299/Molecular-orbitals-of-triplet-oxygen-19-Molecular-orbitals-of-triplet-oxygen-19.png)







0 Response to "38 orbital diagram for oxygen"
Post a Comment