Complete answer: Diagrams representing the arrangement of orbitals in increasing order of their energy levels are known as orbital energy diagrams or energy ...1 answer · Top answer: Hint: Energy of orbitals of hydrogen and hydrogen like atoms which consist of only one electron, depends on the value of principal quantum number only ...
Beryllium (Be) has an atomic mass of 4. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.
In this figure, the element symbol L i is followed by the electron configuration,. An atom of the alkaline earth metal beryllium, with an atomic number of 4 ...

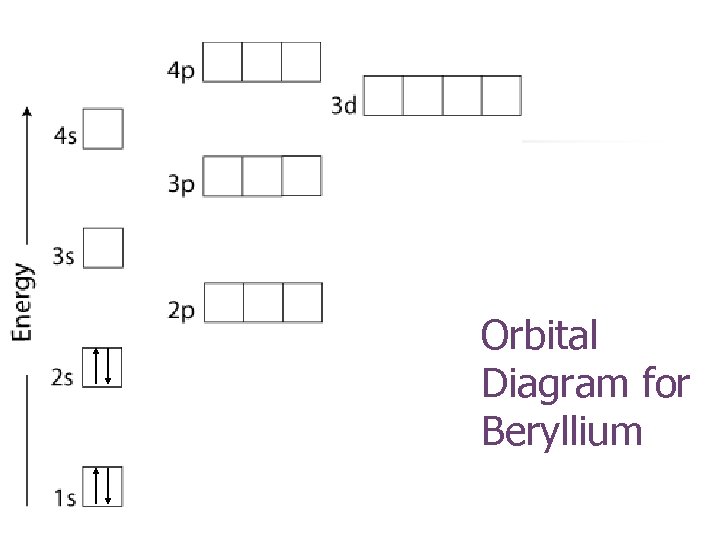
Orbital diagram for beryllium
(i) Be2 molecule: The electronic configuration of Be(Z = 4) is:4 Be 1s2 2s1Be2 molecule is formed by the overlap of atomic orbitals of both beryllium atoms. Rating: 4,4 · 740 votes · Free · Android · Educational
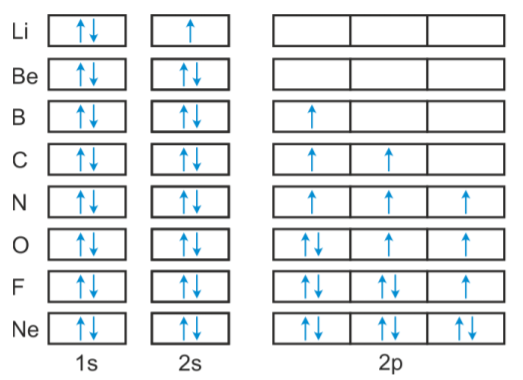
7 Nov 2021 — From the orbital diagram, we can write the electron configuration in an ... The next element is beryllium, with Z = 4 and four electrons.
The orbital diagram for beryllium is shown here. 8 cm-1 (18. KM00 Okay, So in this problem, we have to write the electron configuration for the brilliant atom. Full electron configuration of barium: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2. [12]
Orbital diagram for beryllium.
1 answerWell, the atomic orbital (AO) ordering is quite normal and predictable. BERYLLIUM AO ENERGY ORDERING. Be 's ground-state electron configuration is the one ...
Beryllium is the fourth element with a total of 4 electrons. In writing the electron configuration for beryllium the first two electrons will go in the 1s ...25 Oct 2016 · Uploaded by Wayne Breslyn
4 Jan 2021 — Beryllium Electron Configuration: Beryllium is the fourth atomic number of the periodic table. The symbol of Beryllium is “Be” and it is a ...
The next element is beryllium which has four electrons. The orbital diagram for beryllium is shown here. The electron configuration is 1s 2 2s 2. The fourth electron is placed in the 2s orbital. The energy required to pair the first 2s electron is less than the energy required to place the electron into the 2p orbital.

Be 2+ electron configuration (beryllium ion)

Unmasa dalha: orbital diagram

5.3 electron configuration flashcards | quizlet

See the electron configuration diagrams for atoms of the elements ...

Electronic configuration of beryllium | spdf | trick |chemistry | atomic number #4

Diagram representation of the element beryllium vector image

Electron configuration for beryllium - brainly.in
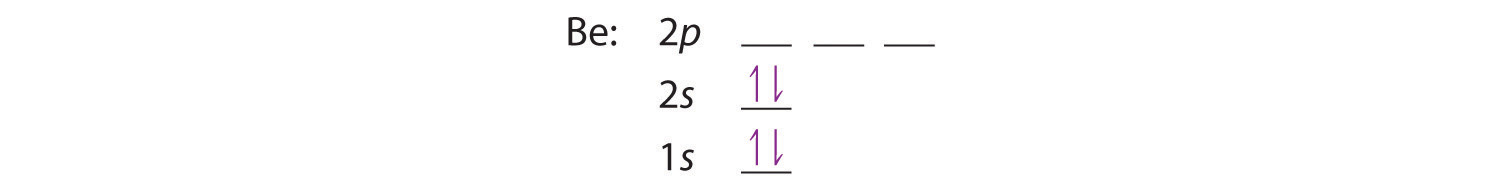
Building up the periodic table

Conventional notation for electronic structure - electronic ...

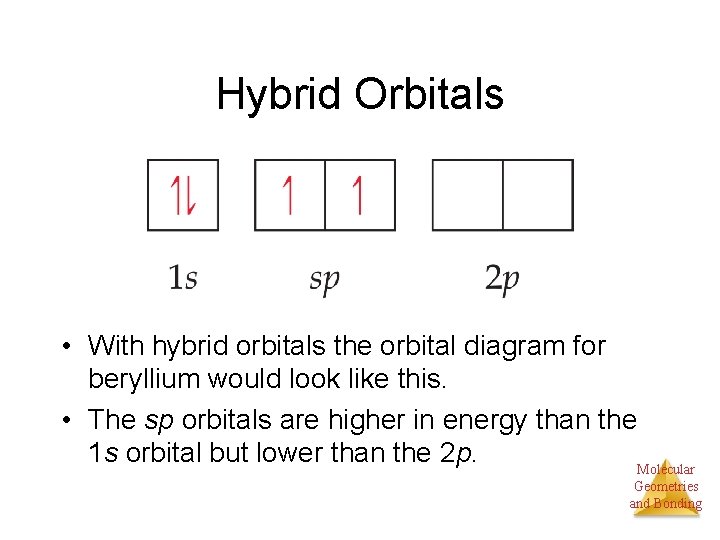
Hybrid orbitals with hybrid orbitals the orbital diagram for ...
Chapter 9 molecular geometries and bonding theories molecular

Write each element's orbital notation and complete electron ...
Orbital energy diagram for beryllium? | socratic
Arrangements of electrons in the orbitals of an atom is called its ...

Diagram representation of the element beryllium - stock ...

Orbital diagrams & electron configurations for atoms and ions

Draw the molecular orbital diagram for:(i) be2(ii) b2 and predict ...

Beryllium electron configuration
Electrons what causes what you are seeing
Write electronic configuration of following elements in form of ...
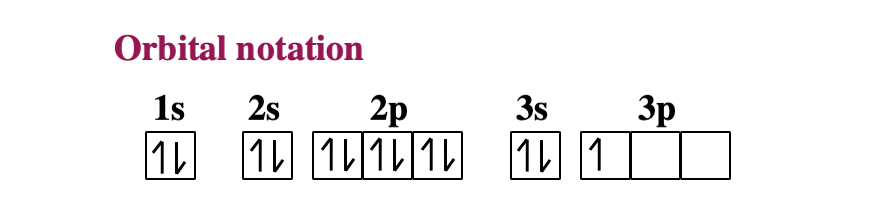
Write each element's orbital notation and complete electron | quizlet
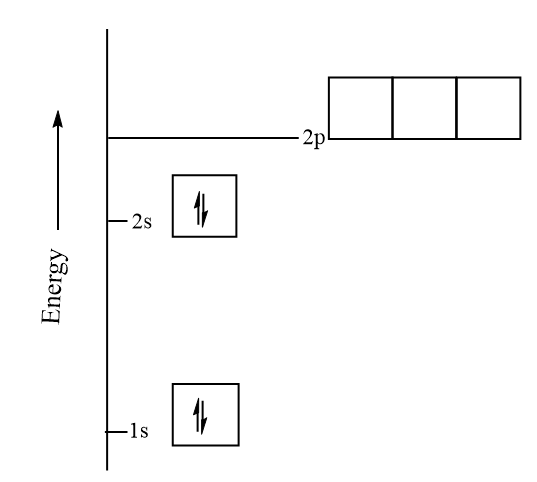
Draw the orbital energy diagram for beryllium class 11 chemistry cbse
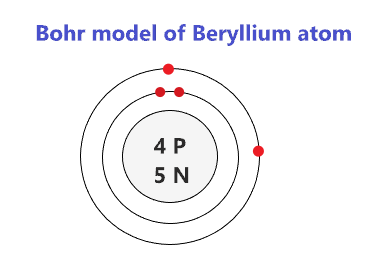
Beryllium bohr model - how to draw bohr diagram for beryllium(be) atom

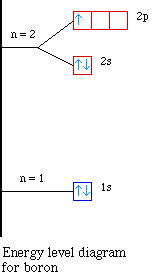
Boron(b) electron configuration with full orbital diagram

1. indicate the number of unpaired electrons present in a ...

Webelements periodic table » beryllium » properties of free atoms
Arrangements of electrons in the orbitals of an atom is called its ...

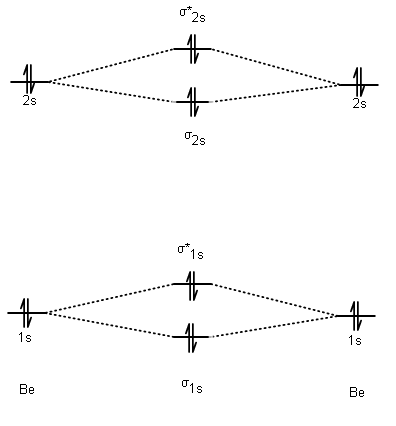
Oneclass: a molecular orbital diagram for beryllium (be) is shown ...
![Solved Review Topics] the References to access important | Chegg.com](https://media.cheggcdn.com/media%2Fb36%2Fb366a881-6786-4550-9679-2288f04044f5%2Fimage)
Solved review topics] the references to access important | chegg.com
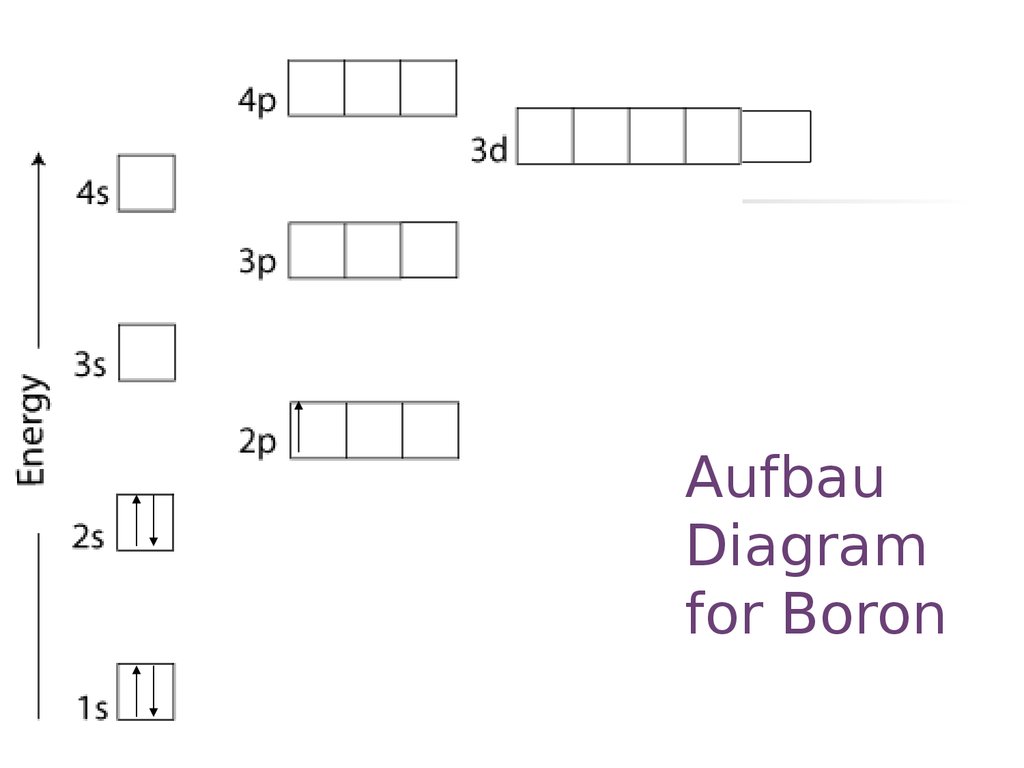
C_electron_config - презентация онлайн

Lithium and beryllium band structures formation through molecular ...
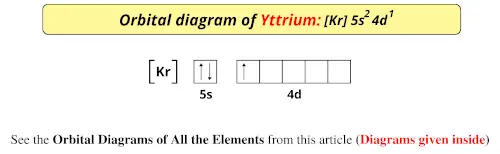
Orbital diagram of all elements (diagrams given inside)

How can we find a beryllium electron configuration (be)

Be beryllium element information: facts, properties, trends, uses ...

Chemistry: the central science, chapter 6, section 8

Solved fill in the orbital energy diagram for the beryllium ...

Solved which of the following orbital diagrams does not | chegg.com

Chemical bonds three basic types ionic electrostatic attraction

Electron configurations and orbital diagrams. principles for ...

Orbital filling diagrams | the cavalcade o' chemistry

Chapter 6, section 8







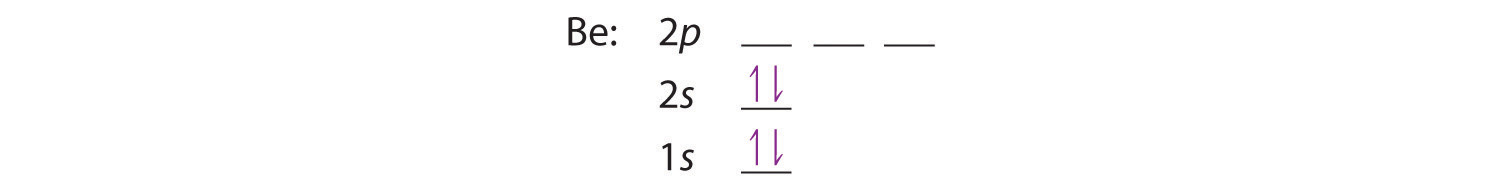



















![Solved Review Topics] the References to access important | Chegg.com](https://media.cheggcdn.com/media%2Fb36%2Fb366a881-6786-4550-9679-2288f04044f5%2Fimage)











0 Response to "42 orbital diagram for beryllium"
Post a Comment