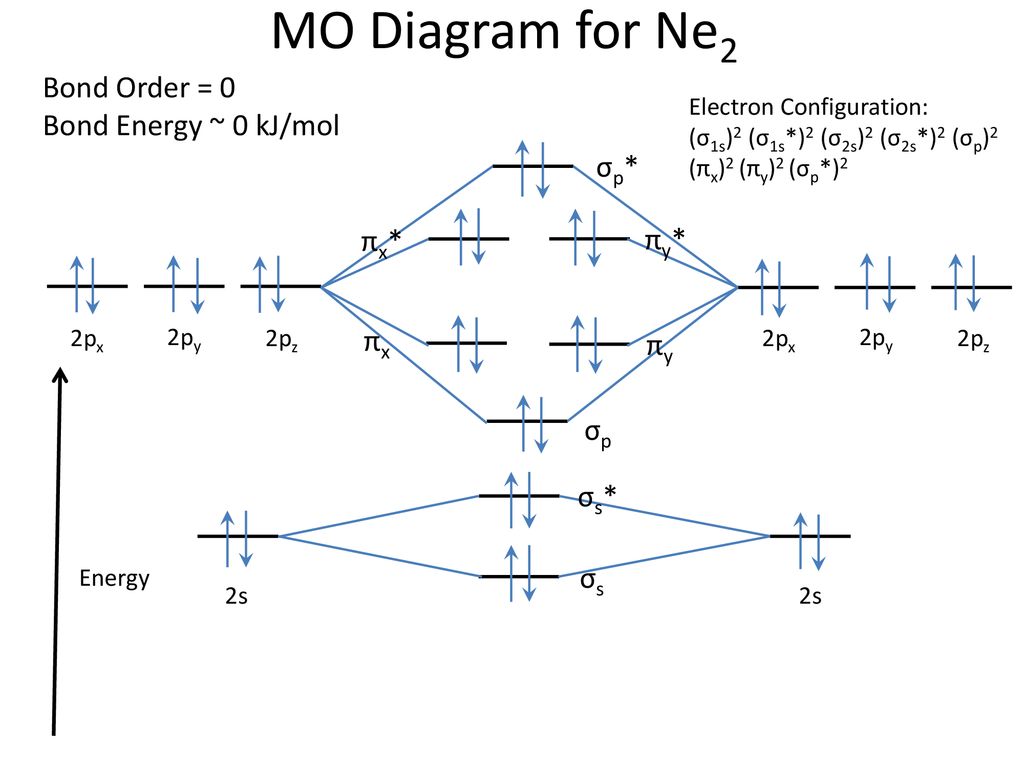
42 ne2 molecular orbital diagram
The MO method for N2+ gives the bond order equal to 2.5. But first, we look at the diagram of molecular orbitals for N2 (the bond order for the nitrogen molecule is 3). the N2+ molecule). That is, the bond order for N2+ is 2.5. Molecular orbital diagram and bond order of fluorine molecule. Get the detailed answer. Bond order in fluorine. 4 posts Page 1 of 1. Mastering Chemistry 9 1212k. Bond Order practice you can also practice MO Theory. Below is a molecular orbital diagram for a fluorine molecule. ... What is the bond order of Be2 F22 F2 and Ne2.
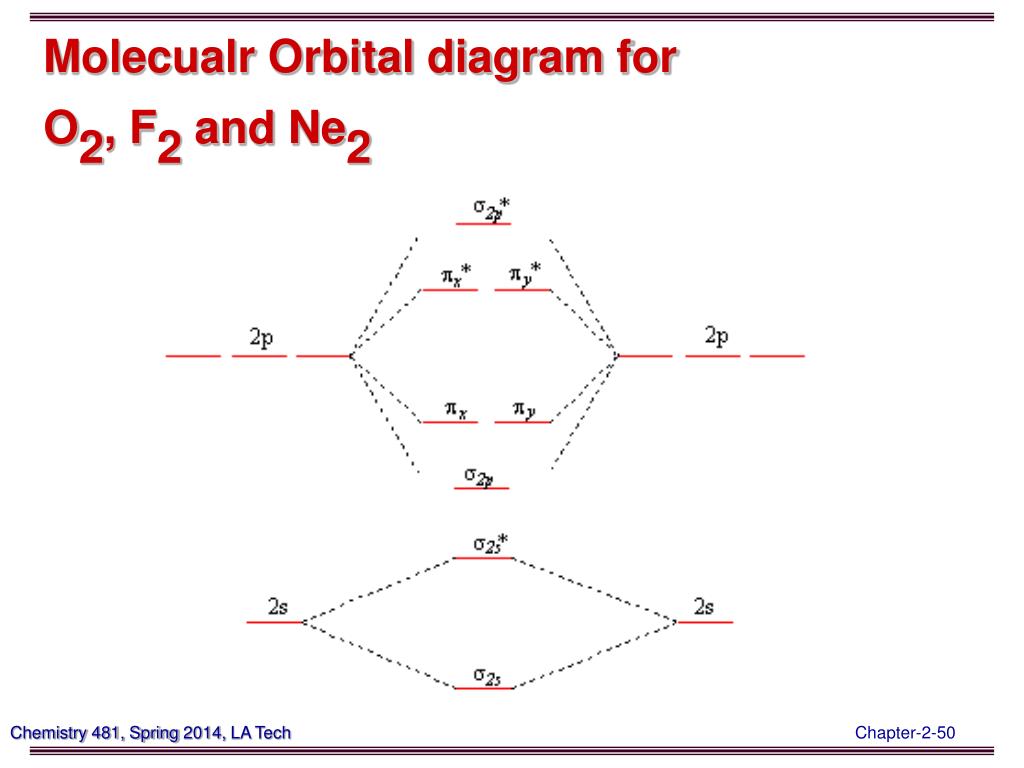
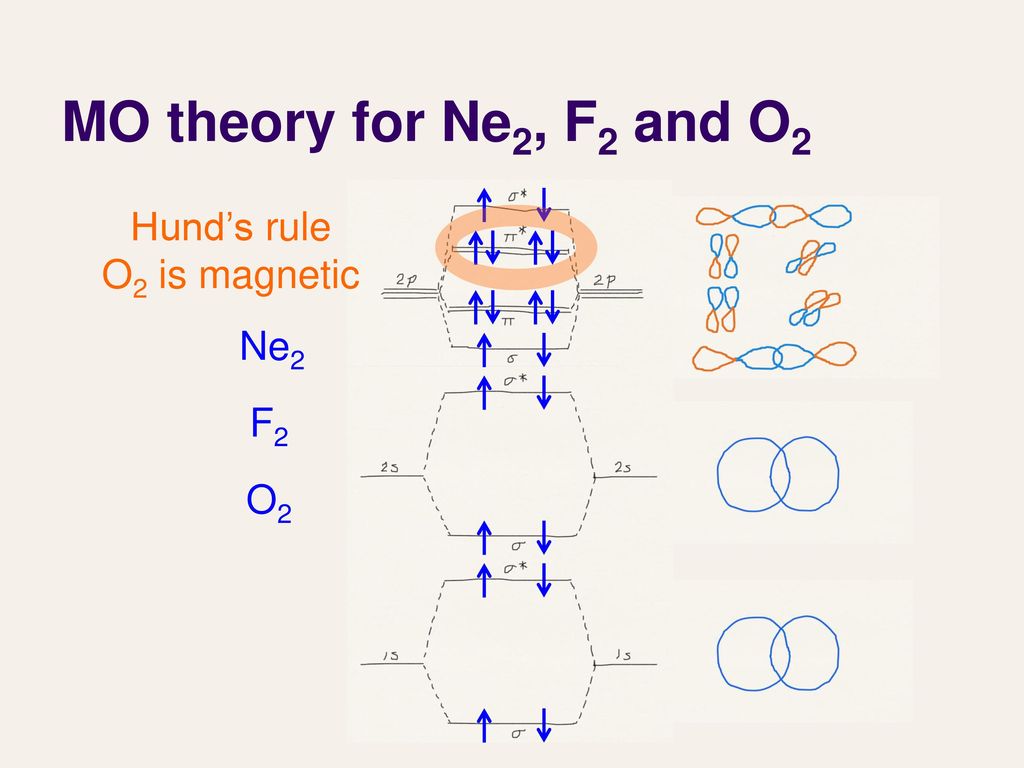
15 F2 Molecular Orbital Diagram. We assume that the electrons would fill the molecular orbitals of molecules like electrons fill atomic we will use this diagram to describe o2, f2, ne2, co, and no. The lowest energy unoccupied molecular orbital is 2p_ (sigma), so that is where the extra electron will be added.

Ne2 molecular orbital diagram
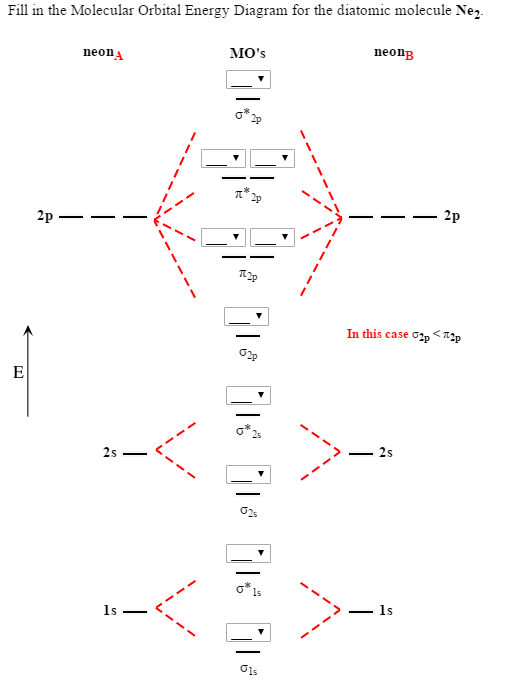
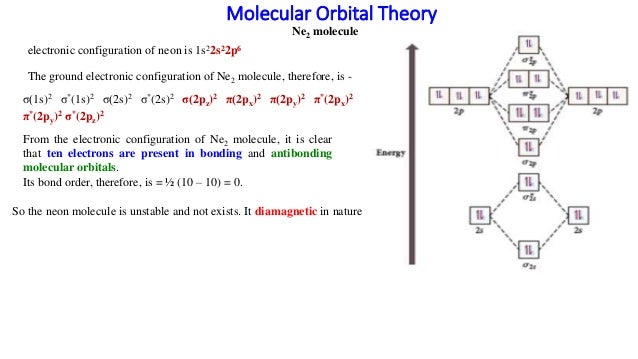
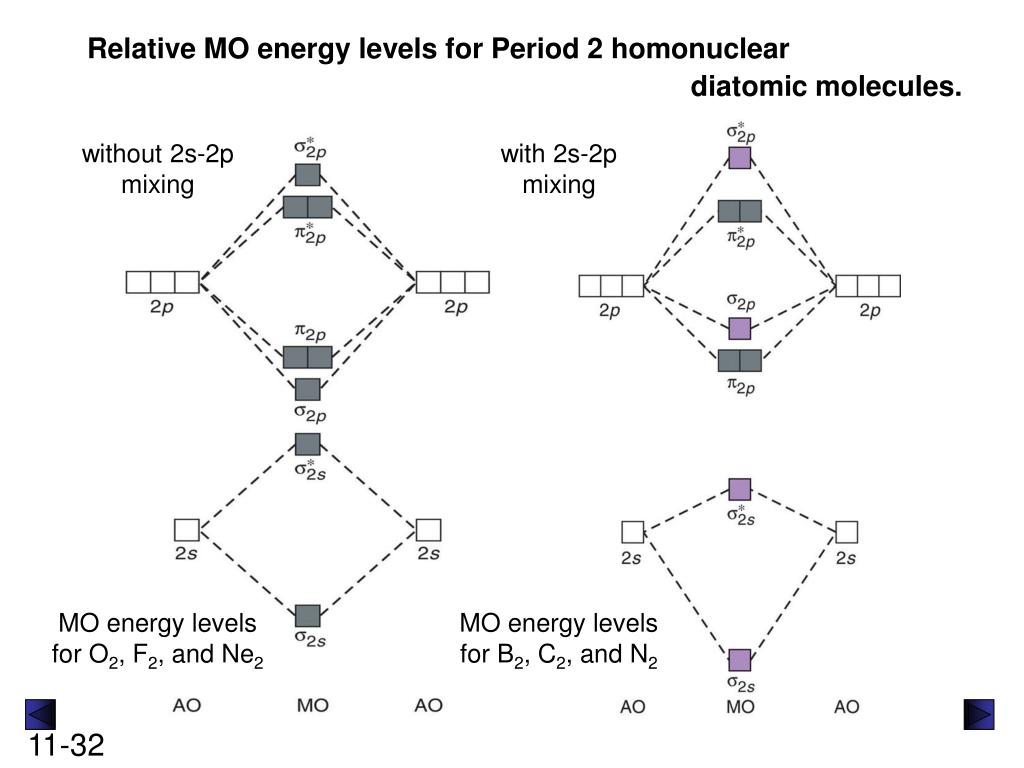
When two orbitals are added, the result is stable bonding molecular orbital and when orbitals are subtracted, it is called unstable anti-molecular bonding (*) which has more energy than the latter one. Considering the energy level diagram, the configuration of N2 is σ1S2, σ *1S2, σ2S2, σ*2S2, π2Px2, π2Py2, σ2Pz1. A molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (lcao) method in particular. Source: i.stack.imgur.com. Period 2 homonuclear diatomic molecules mo diagram co2 bonding. Why is Ne2 unstable? Therefore, formation of this molecule is not possible. According to the molecular orbital theory, the electronic configuration of Ne2 suggests that the number of electrons in bonding orbitals is equal to the number of electrons in the antibonding orbitals. Hence, the bond order is zero or the molecule does not exist.
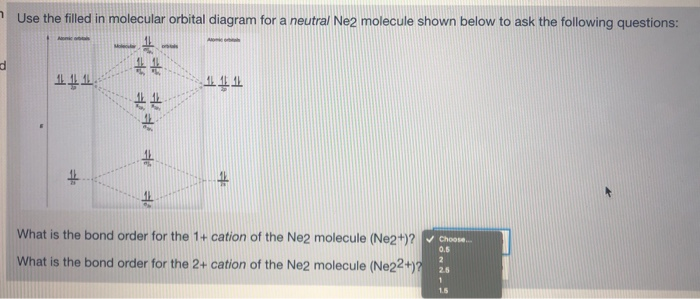
Ne2 molecular orbital diagram. As you can see, Ne2 has all of its orbitals (both bonding and antibonding—labeled with a *) filled. To form the 2 + ion, the uppermost electrons in the sigma* 2p orbital are removed, making it isoelectronic with F2, so it has a bond order of 1 and should be observable, though highly reactive. Molecular Orbital Diagram Of C2, N2 , O2 ,F2. Molecular Orbital Diagram Of C2, N2 , O2 ,F2. Books. Physics. NCERT DC Pandey Sunil Batra HC Verma Pradeep Errorless. Chemistry. ... Molecular Diagram Of O2(2-), F2, Ne2 |Bond Strength #!#Bond Length #!#Bond Order 643505102 000+ 600+ 38:56 ... I3 Lewis Structure, Molecular Geometry, Hybridizat ion, Polarity, and MO Diagram. I3- or triiodide ion is a polyatomic molecule or a charged molecule having a net negative charge of -1. I2 + I- —-> I3-. This is the exergonic equilibrium leading to the for mat ion of the ion where a positive flow of energy happens from the system to the ... According to the molecular orbital theory, the electronic configuration of Ne2 suggests that the number of electrons in bonding orbitals is equal to the number of electrons in the antibonding orbitals. Hence, the bond order is zero or the molecule does not exist.
Question: Complete the molecular orbital diagram for CN. Note that the 1s orbitals are not shown. Identify the bond order of CN. O2 01 OOOOO 25- 0 2s Answer Bank The atomic orbitals on the left side of the molecular orbital diagram are those of The atomic orbitals on the right side of the molecular orbital diagram are those of. An advanced molecular orbital diagram of beh2 beryllium hydride for the inorganic or physical chemistry student. Molecular orbitals and walsh diagram. Walsh correlation diagram is a plot of molecular orbital energy as a function of some systematic change in molecular geometry. Be has 2s and 2p orbitals and it is in the middle. 38 molecular orbital diagram of h2. Written By Chelsea P. Mariano Monday, November 8, 2021 Add Comment. Edit. 3 Feb 2021 — For H2, bond order = 1/2 (2-0) = 1, which means H2 has only one bond. The antibonding orbital is empty. Thus, H2 is a stable molecule. Again, in ... Why is Ne2 unstable? Therefore, formation of this molecule is no possible. According to the molecular orbital theory, the digital configuration that Ne2 argues that the variety of electrons in bonding orbitals is equal to the variety of electrons in the antibonding orbitals. Hence, the shortcut order is zero or the molecule does no exist.
Question: Use Molecular Orbital Theory To Determine Whether He2 Or He2+ Is More Stable. These properties can be explained by the molecular orbital diagram of BN". The bond order of two suggests that the oxygen molecule is stable. Correct option (a) O-2. Diamagnetic Metals + properties give you a broad overview of these metals from multiple angels. Figure 9.7. 3: Molecular Orbital Energy-Level Diagrams for Diatomic Molecules with Only 1 s Atomic Orbitals. (a) The H 2+ ion, (b) the He 2+ ion, and (c) the He 2 molecule are shown here. Figure 9.7. 3 a shows the energy-level diagram for the H 2+ ion, which contains two protons and only one electron. Molecular orbital diagram for carbon dimer c2. Molecular orbital diagram for n2 o2 c2 f2 also h2o. Fill from the bottom up with 8 electrons total. Mo diagram s can be used to deduce magnetic properties of a molecule and how they change with ionization. O2 2 Molecular Orbital Diagram Fabulous Electron Molecular Orbital. Molecular orbital diagram for c2.This video shows the mo diagrams of the c2 ... This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H 2 +. Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as is the case for atomic orbitals.
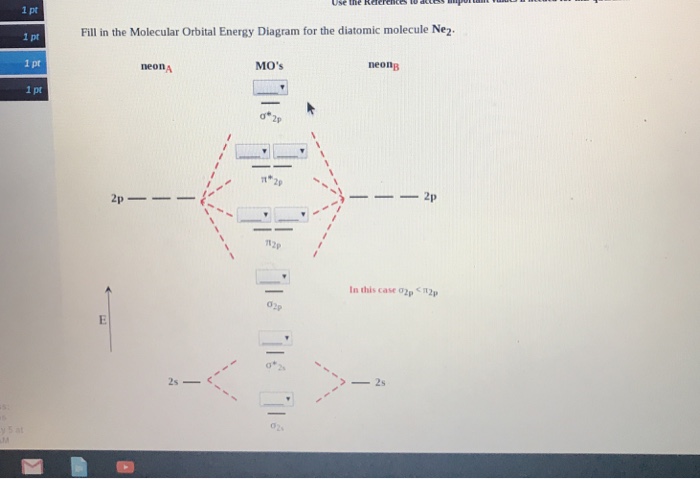
Molecular orbital diagram for ne2 2. 2s. I know ill need a 2p orbital but theres orbital mixing going on so i have 2 choices. Wiki User Answered 2012-03-05 06:41:57. Because According to molecular orbital theory O 2 + has 15 electrons &it has one electron in antibonding orbital. Side by Side Comparison - Homo vs Lumo in Tabular Form 5.
Molecular orbital of ne2 Get the answers you need, now! venketeshavs5549 venketeshavs5549 27.02.2021 Chemistry Secondary School answered Molecular orbital of ne2 2 See answers Advertisement Advertisement Brainly User Brainly User Molecular orbital diagram of •Neon atom has 10 electrons and its electronic configuration is considered, it has ...
1. Problem: Draw MO energy diagram s for the molecular ions H2+ and H Since both molecular ions have a bond order of 1/2, they are approximately equally.Solution: Construct the molecular orbital diagram for He2 + and then identify the bond order. Problem Construct the molecular orbital diagram for He 2 + and then identify the bond order. How to write simple Molecular Orbital Diagram s and ...
a) The point at which the solid, liquid and gaseous phases for a substance co-exist. b) The triple point exists for a substance at a specific temperature and pressure. c) The triple point exists at a single temperature and is not related with pressure. d) The system must be closed so that no vapor can escape.
Molecular orbital diagram for ne2 2 Molecular orbital diagram for ne2 2 ... 25 Apr 2021 — Ti tanium atoms have 22 electrons and the shell structure is 2.8. 10.2. The ground state electron configura ti on of ground state gaseous neutral ... 06-05-2021 · For each of the following, ...
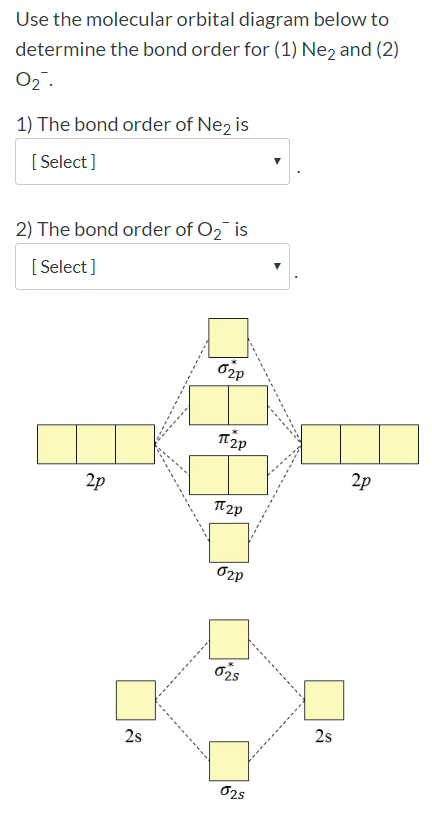
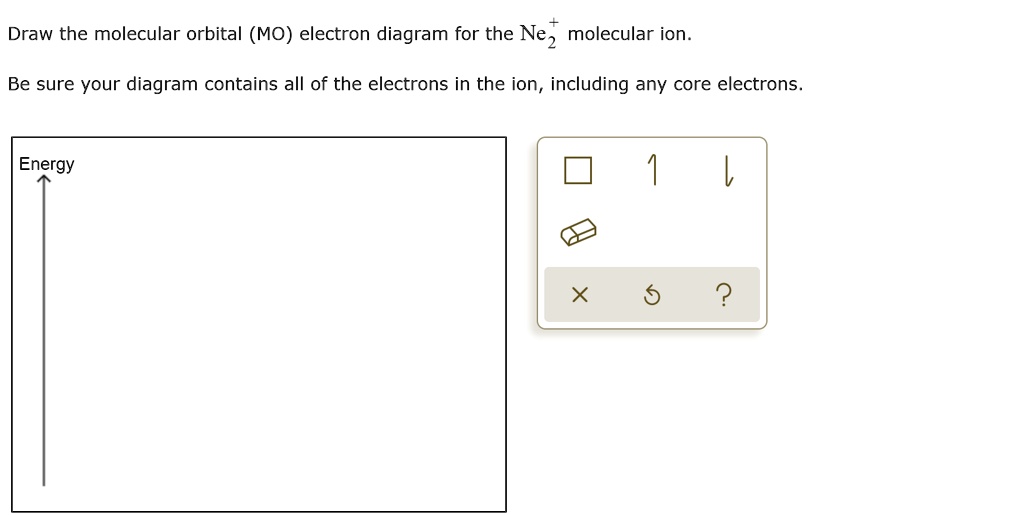
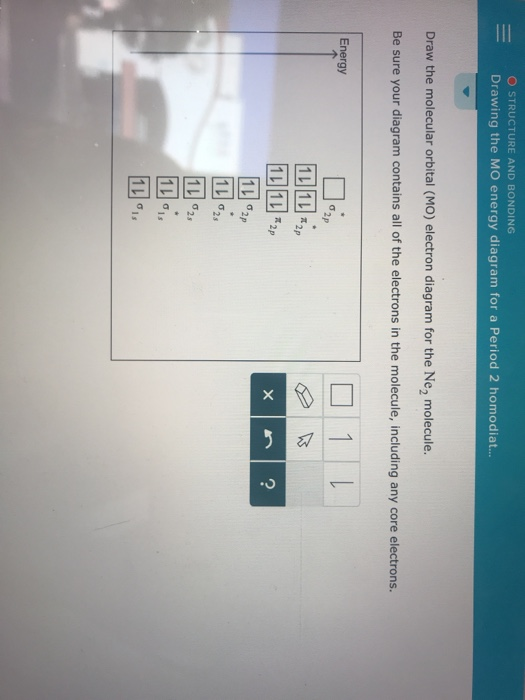
Molecular orbital diagram for ne2. Answer to draw the molecular orbital diagram for ne2 and determine if the bond between the two atoms will be stable. Give each mo an appropriate label. For ne2 construct three molecular orbital diagrams one each for the neutral molecule the 1 cation and the 1 anion. The other is for after nitrogen starting at.
Why is Ne2 unstable? Therefore, formation of this molecule is not possible. According to the molecular orbital theory, the electronic configuration of Ne2 suggests that the number of electrons in bonding orbitals is equal to the number of electrons in the antibonding orbitals. Hence, the bond order is zero or the molecule does not exist.
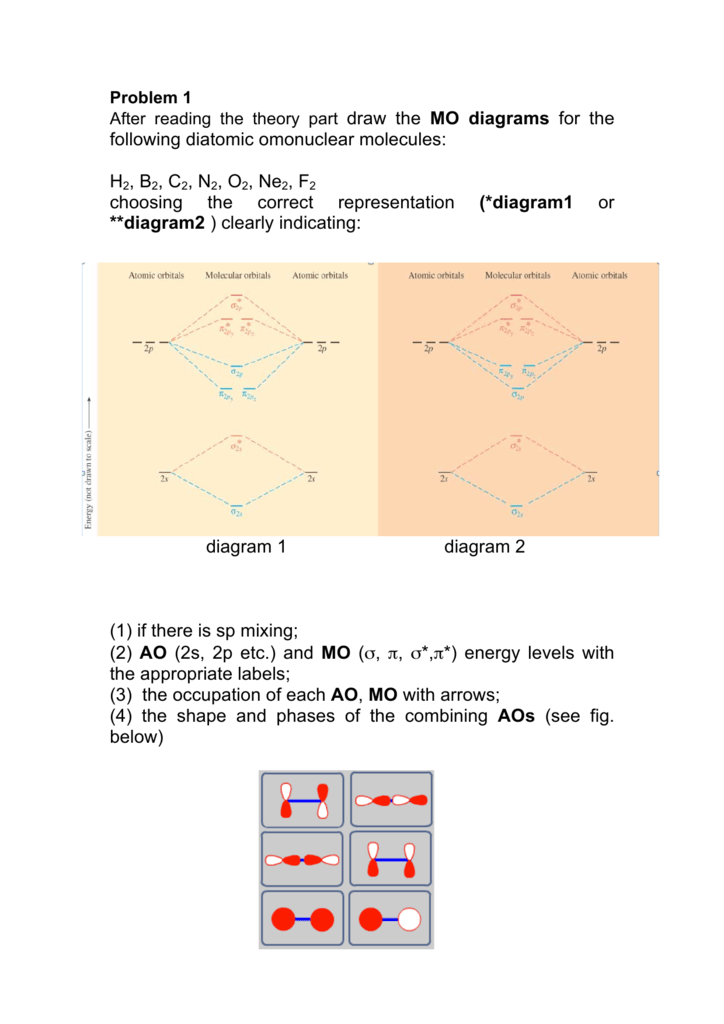
A molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (lcao) method in particular. Source: i.stack.imgur.com. Period 2 homonuclear diatomic molecules mo diagram co2 bonding.
When two orbitals are added, the result is stable bonding molecular orbital and when orbitals are subtracted, it is called unstable anti-molecular bonding (*) which has more energy than the latter one. Considering the energy level diagram, the configuration of N2 is σ1S2, σ *1S2, σ2S2, σ*2S2, π2Px2, π2Py2, σ2Pz1.


















0 Response to "42 ne2 molecular orbital diagram"
Post a Comment