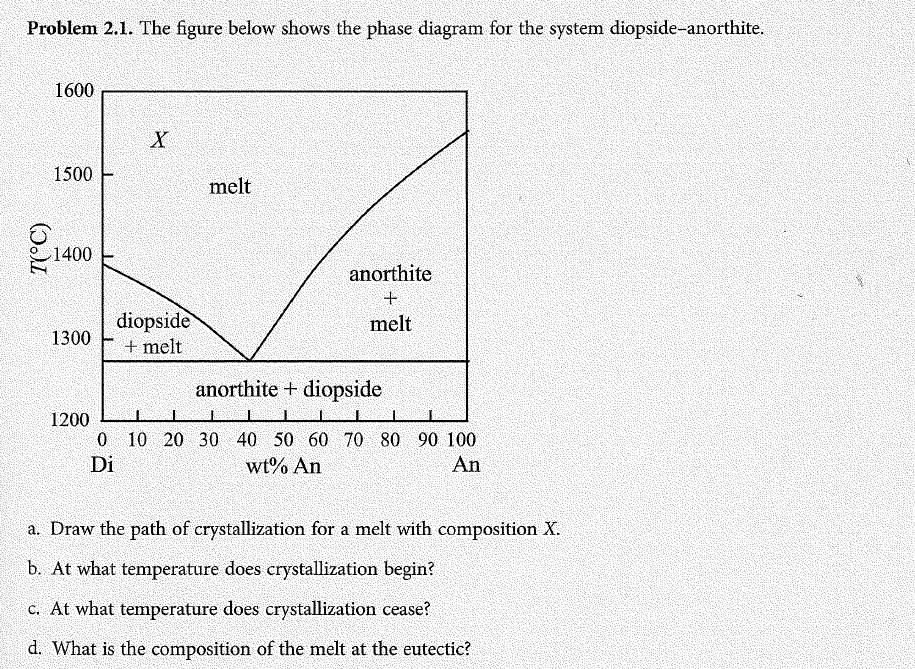
42 diopside anorthite phase diagram
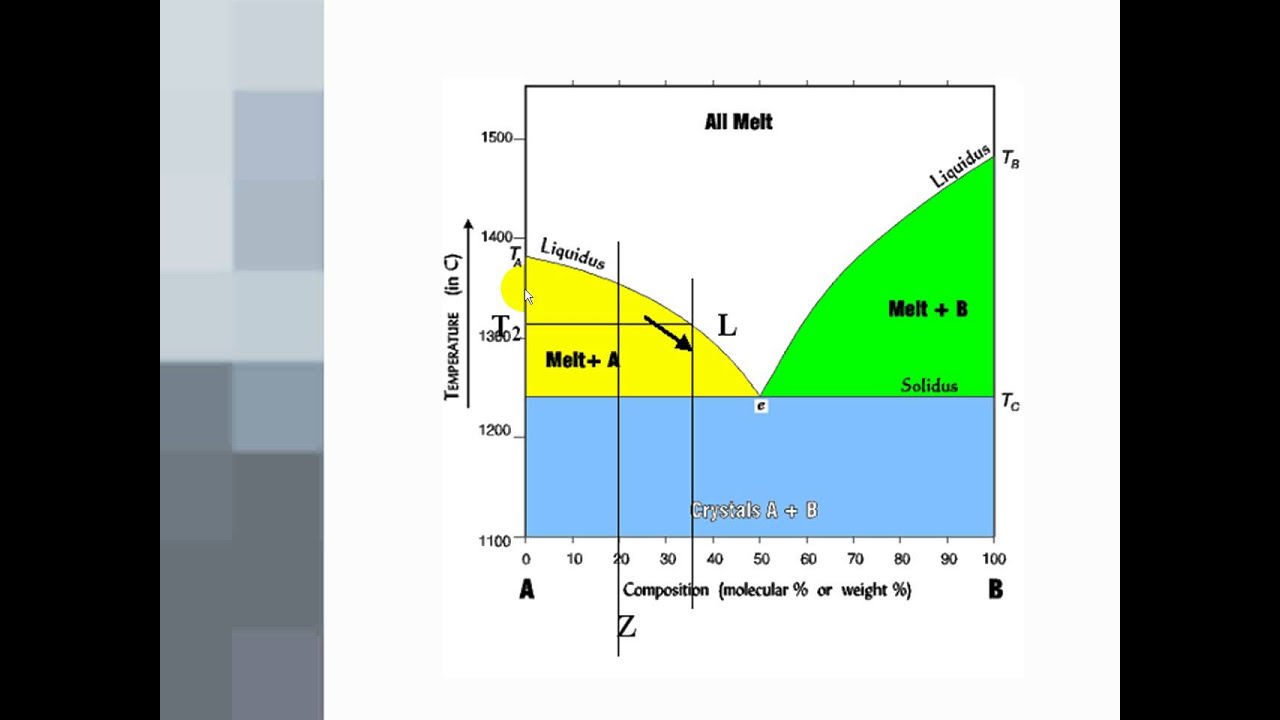
K Anorthite-diopside system: both end members are congruently melting and compatible; system has a eutectic. End product of cooling is individual crystals of diopside and anorthite, in the same wt. % ... phase diagrams is found in chapter 10 of the book by Bloss (Crystallography and
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
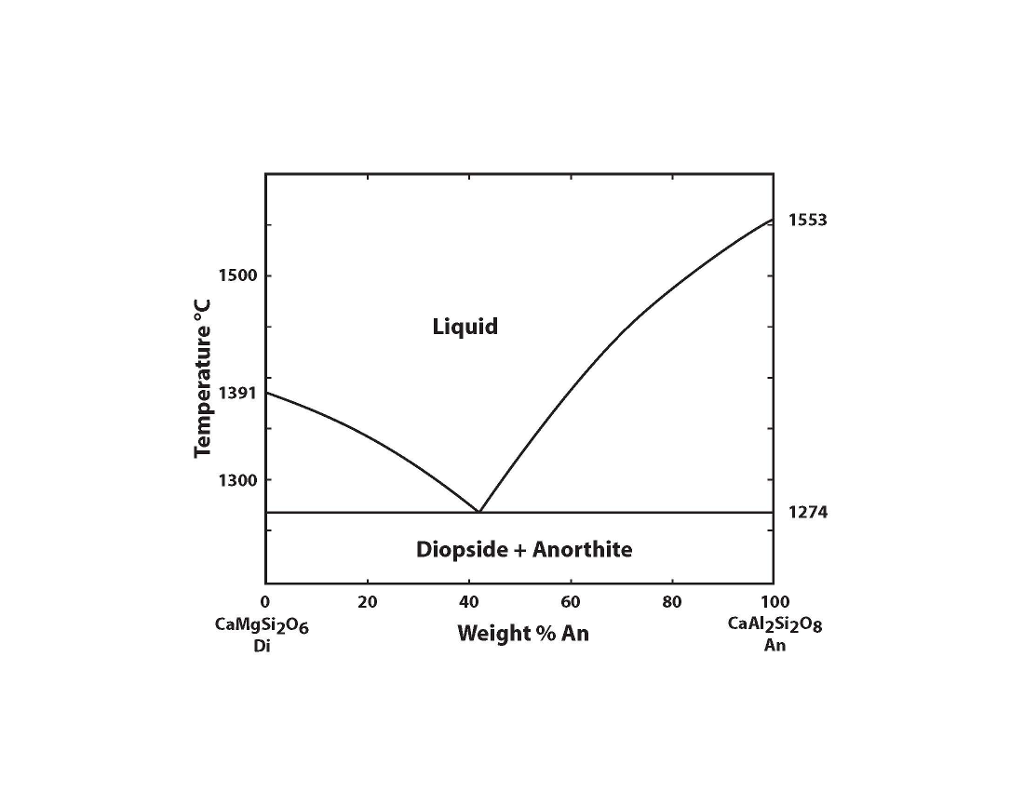
Diopside Anorthite Temp. Vol. % Melt Melt Comp. Vol. % Solid Solid Comp. T1 T3 T2 T4 100 64 57 0 70An+30Di 53An+47Di 47An+53Di NA 20 40 60 80 0.0 36 43 100 N/A An An 70An+30Di ... Binary Eutectic+Subsolidus Phase Diagram Equilibrium crystallization of composition M Or-rich feld. + MELT Ab-rich feld. + MELT Two feldspars Or-rich feld. Ab-rich ...
Diopside anorthite phase diagram
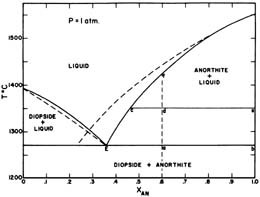
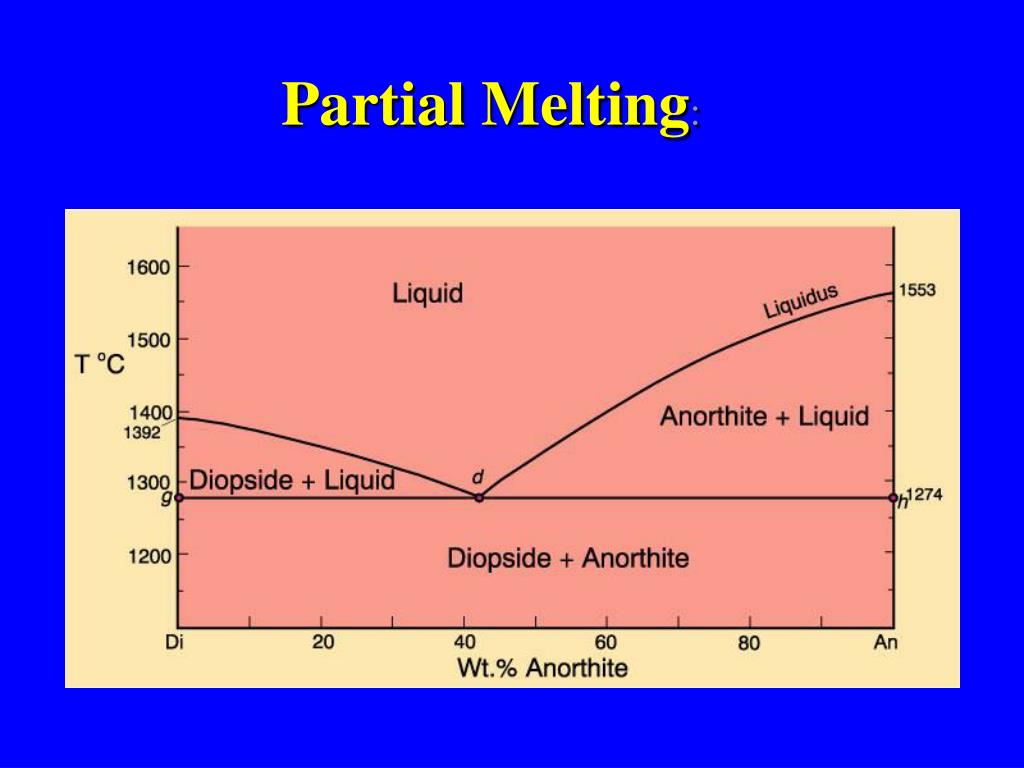
Both anorthite and diopside melt in eutectic proportions (which is richer in anorthite than the bulk composition b). Liquid produced has eutectic composition · Mixture stays at constant T until ALL anorthite has melted · Once last bit of anorthite has melted, still have diopside, T can rise again.
Anorthite - Diopside System • This system contains two congruently melting compounds anorthite and diopside • Congruently Melting Compound – A compound which when heated melts directly to a liquid of its own composition • eg. Solid anorthite has a composition of CaAl 2Si 2O 8 which when heated melts to a liquid with the same composition ...
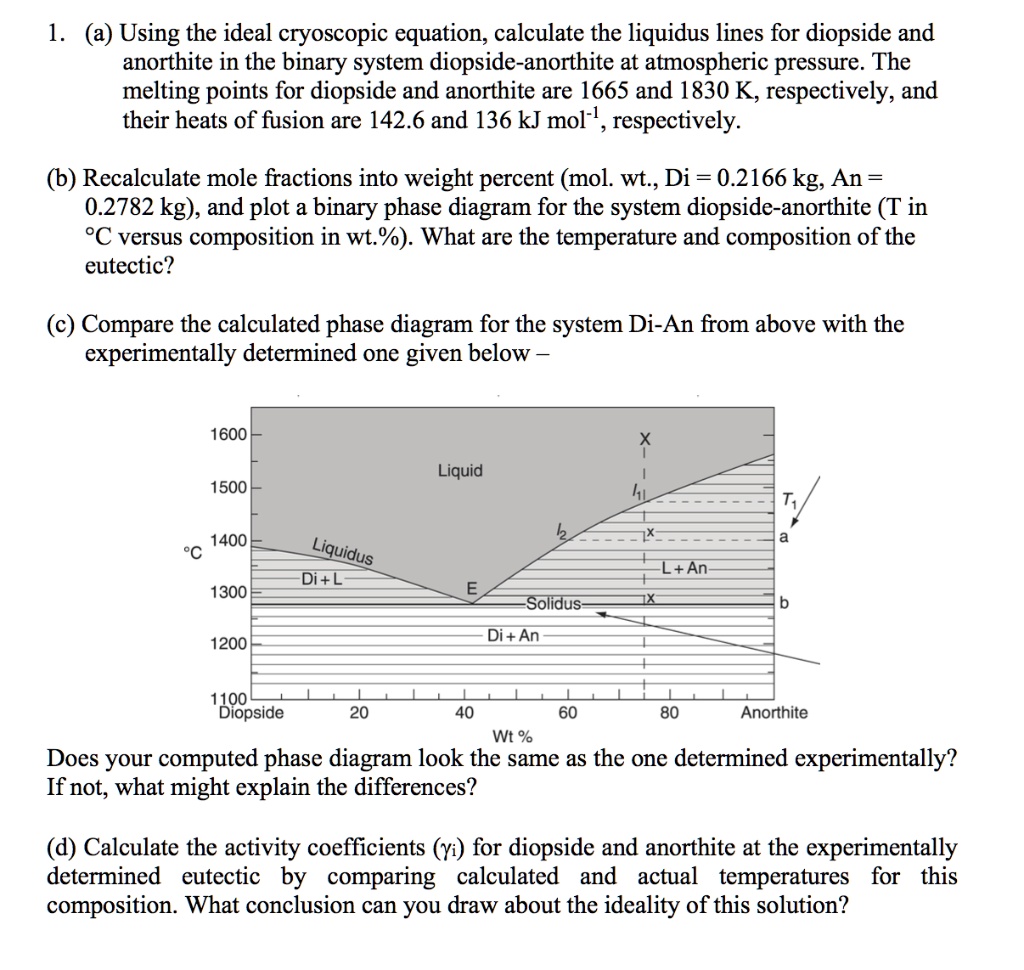
Binary Phase Diagrams 1 Phase Diagram Exercise for Binary Systems Problem 1. Figure 1 is the binary phase diagram for the Anorthite-Diopside system. With this diagram calculate the following assuming equilibrium crystallization: Composition X %Melt Comp. of Melt %Solid Comp. Solid
Diopside anorthite phase diagram.
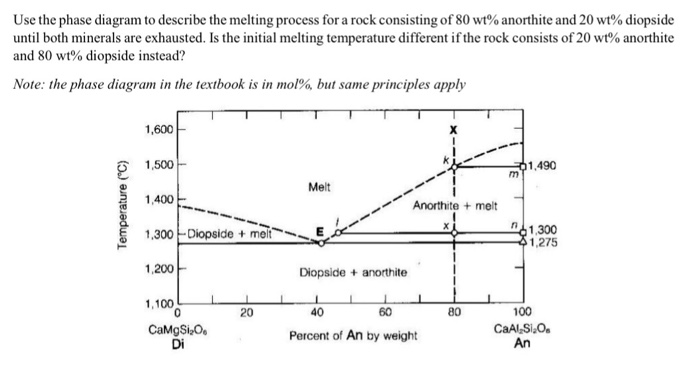
anorthite and 88% diopside. If we heat it, melting commences at point A, about 1275 oC (equal to the eutectic temperture). The first liquid has the composition of the eutectic (point E). It forms as anorthite and diopside melt together. Temperature will not rise above 1275 oC until all the anorthite has melted. Above the eutectic temperature, the
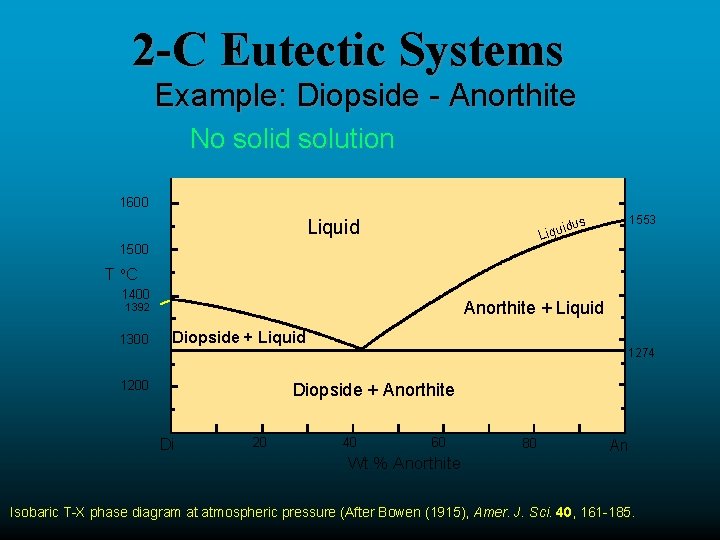
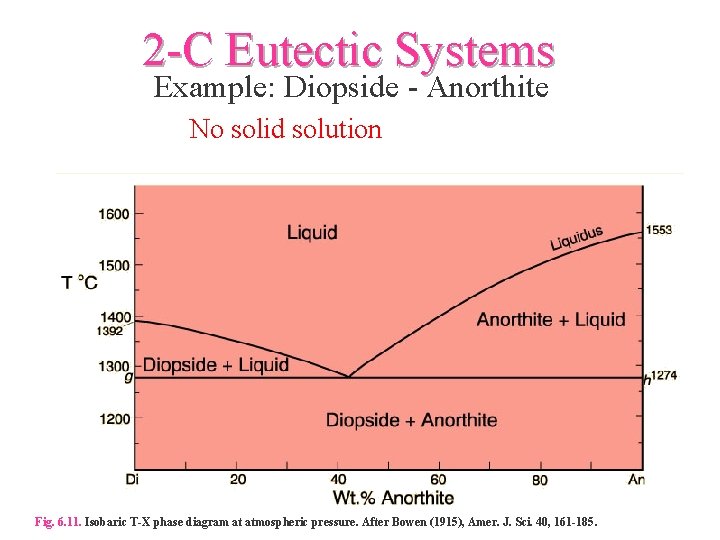
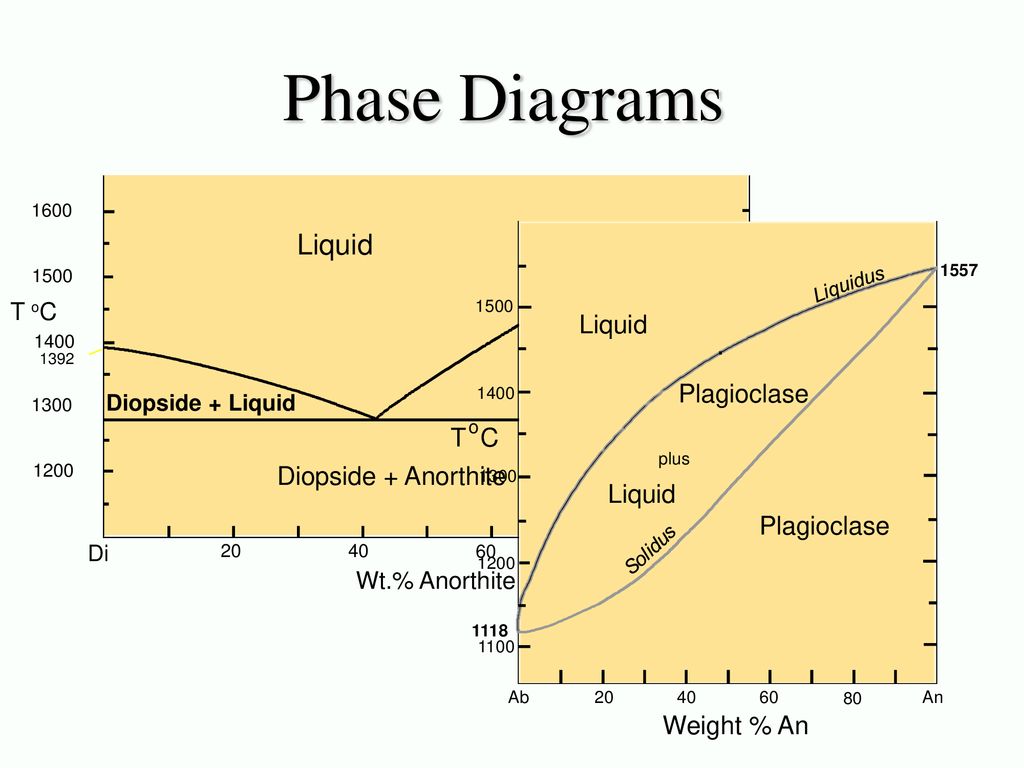
Example: Diopside - Anorthite No solid solution between Diopside and Anorthite Fig. 6-11. Isobaric T-X phase diagram at atmospheric pressure. After Bowen (1915), Amer. J. Sci. 40, 161-185. 2 This simple system is analogous to a basaltic magma crystallizing pyroxene and plagioclase.
The relationship of phases developed during the crystallization of certain glasses within the system diopside-anorthite-akermanite with and without Cr 2 O 3 was studied under different conditions of heat treatment. The mineral phases developed in the Cr 2 O 3-free glasses after heat treatment were in good accordance with the equilibrium phase diagram.The phase relationships were greatly ...
Example: Diopside - Anorthite No solid solution Isobaric T-X phase diagram at atmospheric pressure. After Bowen (1915), Amer. J. Sci. 40, 161-185. Cooling from composition a: bulk composition = An70 First crystal forms at 1455oC (point b) with a compositon of pure An • Cooling continues as Xliq varies along the liquidus






![PDF] Liquidus phase relations on the join diopside-forsterite ...](https://d3i71xaburhd42.cloudfront.net/ed00f100fe3574fd62ab14c1a154edace8ca157c/2-Figure2-1.png)



0 Response to "42 diopside anorthite phase diagram"
Post a Comment