38 fill in the orbital energy diagram for the fluoride ion.
January 22, 2021 - Check here the Fluorine Electron Configuration with Orbital Diagram. The symbol of Fluorine and other infomation of F also given here. Fill in the orbital energy diagram for the chromium(II) ion The lowest E levels are already filled m for you Fill m the orbital energy diagram for the fluoride ion ... This problem has been solved! ... Fill in the orbital energy diagram for the chromium(II) ion The lowest E levels are already ...
Exercise 5. P. 3: The Peroxide Ion. Use a qualitative molecular orbital energy-level diagram to predict the electron configuration, the bond order, and the number of unpaired electrons in the peroxide ion (O 22− ). Draw the MO diagram for hydroxide ion. Draw the MO diagram for hydrogen fluoride.
Fill in the orbital energy diagram for the fluoride ion.
Answer: This just shows energy levels so let's take this a step further. Atomic Electron Configurations And I'm not having any luck but if you go to this site, you should be about to see what the 1s, 2s, 2px, 2py, 2pz, and 3s orbitals look like together. Jmol orbital structures If not, see what... Fill in the orbital energy diagram for the fluoride ion. 2p El 2s 1s 4 more group attempts remaining Submit Answer Retry Entire Group ; Question: Fill in the orbital energy diagram for the fluoride ion. 2p El 2s 1s 4 more group attempts remaining Submit Answer Retry Entire Group How to Write Electron Configurations. Step-by-step tutorial for writing the Electron Configurations.
Fill in the orbital energy diagram for the fluoride ion.. The pairwise combination of AOs used to construct the fluorine molecular orbital diagram. A comparison between fluorine's MO diagram and its Lewis dot diagra... The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the remaining five electrons. Therefore the Chlorine electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 5. August 15, 2020 - We use \(Z_{eff}\) instead of Z to mean that we have to modify the atomic number to get an effective atomic charge for the nucleus. Since we are dealing with approximate values, one may use Z directly. The 1s orbital energy level is -13.6 eV for hydrogen atoms, measured as the ionization energy ... Chemistry questions and answers. Fill in the orbital energy diagram for the fluoride ion. - - E2 - Fill in the orbital energy diagram for the chromium (III) ion. 3d E3s- AV AV AV 2p The lowest E levels are already filled in for you. Question: Fill in the orbital energy diagram for the fluoride ion.
Click on the CO molecular orbitals in the energy level diagram to display the shapes of the orbitals. Explore bonding orbitals in other small molecules. Hydrogen | Fluorine | Nitrogen | Hydrogen Fluoride | Carbon Monoxide | Methane | Ammonia | Ethylene | Acetylene | Allene | Formaldehyde | Benzene Fluorine (F) has an atomic mass of 9. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. Module Two Chem 101 Problems. Carbon dioxide is a _____ compound composed two types of _____ atoms. Carbon dioxide is a molecular compound composed of two oxygen atoms with covalent double bonds to a central carbon atom. Both C and O are to the right of the "staircase" on the periodic table, as nonmetals. Classify the following compounds as ... Orbital diagrams are pictorial descriptions of the electrons in an atom. Three rules are useful in forming orbital diagrams. According to the Auf Bau Principle, each electron occupies the lowest energy orbital. Orbital diagrams are a pictorial description of electrons in an atom.
Molecular orbital diagram for o2- ion 1918 (Venn's diagram is from 1904), named for English logician John Venn (1834-1923) of Cambridge, who explained them in the book "Symbolic Logic" (1881). 1834, introduced by English physicist and chemist Michael Faraday (suggested by the Rev. William Whewell, English polymath), coined from Greek ion ... Question: Fill in the orbital energy diagram for the fluoride ion. 2p E 2s 1s. This problem has been solved! See the answer ... Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. Electron Configurations The Pauli exclusion principle says that all electrons in an atom have to have a unique set of quantum numbers. NO duplicates! It's like a serial number for electrons, except we use n, ℓ, m ℓ, and m s.. The aufbau principle tells us to "build up" from the bottom of the energy well to the top. Pour water in a bucket and it fills from the bottom up - same idea.
Answer (1 of 8): O^2- refers oxide ion. The atomic number of oxygen is 8. The electronic configuration of Oxygen is 1s^2 2s^2 2p^4 The charge of oxide ion is —2, which means an Oxygen atom gained 2 extra electrons. So,this two extra electrons will be attached the last shell's orbital (2p). S...
Problems 1 and 2: orbital energy diagram - ions Problem3: Electron Configurations for Main Group Ions Problem 4: Electron Configurations of Ions Problem1 Fill in the orbital energy diagram for the nitride ion using the following key:
However, for atoms with three or fewer electrons in the p orbitals (Li through N) we observe a different pattern, in which the σp orbital is higher in energy than the πp set. Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting electrons from the diagram ...
An important property of the ethene molecule, and alkenes in general is the existence of a high barrier to rotation about the C=C which tends to hold the molecule flat. For the energy diagram and pictorial view of the orbitals - please see below: ... A simple diatomic molecule is Hydrogen fluoride. ...
Question: Fill in the orbital energy diagram for the oxide ion. 2p 1s Submit Answer Retry Entire Group 5 more group attempts remaining Necd
Question: Fill In The Orbital Energy Diagram For The Copper(II) Ion. 3d 4s 3p E 3s AV NN 2p AN 2s AV 1s The Lowest E Levels Are Already Filled In For You. Submit Answer Retry Entire Group 9 More Group Attempts Remaining Ker Fill In The Orbital Energy Diagram For The Fluoride Ion.
It will gain 2 electron(s) to obtain the nearest noble gas configuration and form an anion with a charge of -2 Fill in the orbital energy diagram for the fluoride ion using the following key: u = electron with spin "up" d = electron with spin "down" Always start with spin up.. .
Frontier molecular orbital theory. In frontier molecular orbital theory (FMO), a reaction is when a filled orbtial → empty orbital. In the example above, the fluoride ion has four filled non-bonding orbitals, denoted n F (n for non-bonding, F for fluorine). BF 3 has an empty non-bonding orbital on boron dubbed n B.
In this video we will write the electron configuration for F-, the Fluoride ion. We’ll also look at why Fluorine forms a 1- ion and how the electron configur...
In this video we will write the electron configuration for F-, the Fluoride ion. We’ll also look at why Fluorine forms a 1- ion and how the electron configur...
Transcribed image text: Fill in the orbital energy diagram for the fluoride ion E2 - 24 ZA 4A SA 6A 7A He Be NO 2 Na Mg 3B 4B 5B 6B7B8B1B2B Al Si K Ca Sc Ti ...
Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom
Chemistry questions and answers. Fill in the orbital energy diagram for the copper (II) ion. 3d 4s 3p E 3s AV NN 2p AN 2s AV 1s The lowest E levels are already filled in for you. Submit Answer Retry Entire Group 9 more group attempts remaining Ker Fill in the orbital energy diagram for the fluoride ion. 2p E 2s 1s 9 more group attempts ...
Figure 10.5. 3: Molecular Orbital Energy-Level Diagrams for Diatomic Molecules with Only 1 s Atomic Orbitals. (a) The H 2+ ion, (b) the He 2+ ion, and (c) the He 2 molecule are shown here. Part (a) in Figure 10.5. 3 shows the energy-level diagram for the H 2+ ion, which contains two protons and only one electron.
Orbital Diagram For Aluminum. A box, line, or circle, is drawn to represent each orbital in the electron configuration (using the Aufbau Principle to order the orbitals and hence. So that's why we needed three florins in order for each of them to accept one of aluminum surveillance electrons. Schematic molecular-orbital diagram showing the ...
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation
An atom of boron (atomic number 5) contains five electrons. The n = 1 shell is filled with two electrons and three electrons will occupy the n = 2 shell. Because any s subshell can contain only two electrons, the fifth electron must occupy the next energy level, which will be a 2p orbital.
Electron Configurations. The content that follows is the substance of General Chemistry Lecture 26. In this lecture we continue the discussion of Quantum Numbers and their use in Electron Configurations as well as the relationship of electron configuration to the periodic properties of the elements.
Question: Fill in the orbital energy diagram for the chromium(II) ion The lowest E levels are already filled m for you Fill m the orbital energy diagram for the fluoride ion. This problem has been solved! See the answer See the answer See the answer done loading. Show transcribed image text
So the electron configuration of potassium will involve 19 electrons. The full electron configuration of potassium is "1s"^2"2s"^2"2p"^6"3s"^2"3p"^6"4s"^1". The noble gas notation is "[Ar]4s"^1". The following orbital diagram shows the increase in energy from one energy sublevel to the next, but you can write them on the same level horizontally,
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
Transcribed image text: Fill in the orbital energy diagram for the chromium(II)ion. 3p- El 35 The lowest Elevels are already filled in for you. (Relerences Use the References to access important values if needed for this question.
Chem 101 Exam 2 Notes. the orbital with n=1, l=0, and m sub 1=0; 1 is value of n (1 sublevel) and s specifies that l=0; high dot density near nucleus indicates higher probability density for electron there; as you move away from nucleus, the probability density decreases; sphere. Nice work!
Transcribed image text: Fill in the orbital energy diagram for the fluoride ion. t remaining BA 3A 4A SA SA ZA LUBO BC NOF MMS 3B 4B 58 68 7B 881B 2B AL Si ...
February 3, 2018 - Answer (1 of 6): Here is the solution, > * For O2 molecule, > * For F2 molecule, Thanks for reading.
F^- : 1s^2 2s^2 2p^6 alternatively: F^- : [Ne] Elemental Fluorine has an electron configuration of 1s^2 2s^2 2p^5 and needs 1 more electron to complete its 2p orbital which it will acquire in formation of the fluoride ion. Thus it gains an electron when forming the fluoride ion, and becomes isoelectronic to neon.
Fluorine (F) Electron Configuration with Full Orbital Diagram. Fluorine electron configuration is 1s 2 2s 2 2p 5. The symbol for fluorine is F. The period of fluorine is 2 and it is a p-block element. The electron configuration of fluorine (F) and the orbital diagram is the main topic of this article.
Fill in the orbital energy diagram for silicon. 3p - Submit Answer Retry Entire Group 9 more group attempts remaining [Review Topics] [References) Use the References to access important values if... Fill in the orbital energy diagram for the iron(III) ion. 3d 4s 3p 2p 2s 1s...
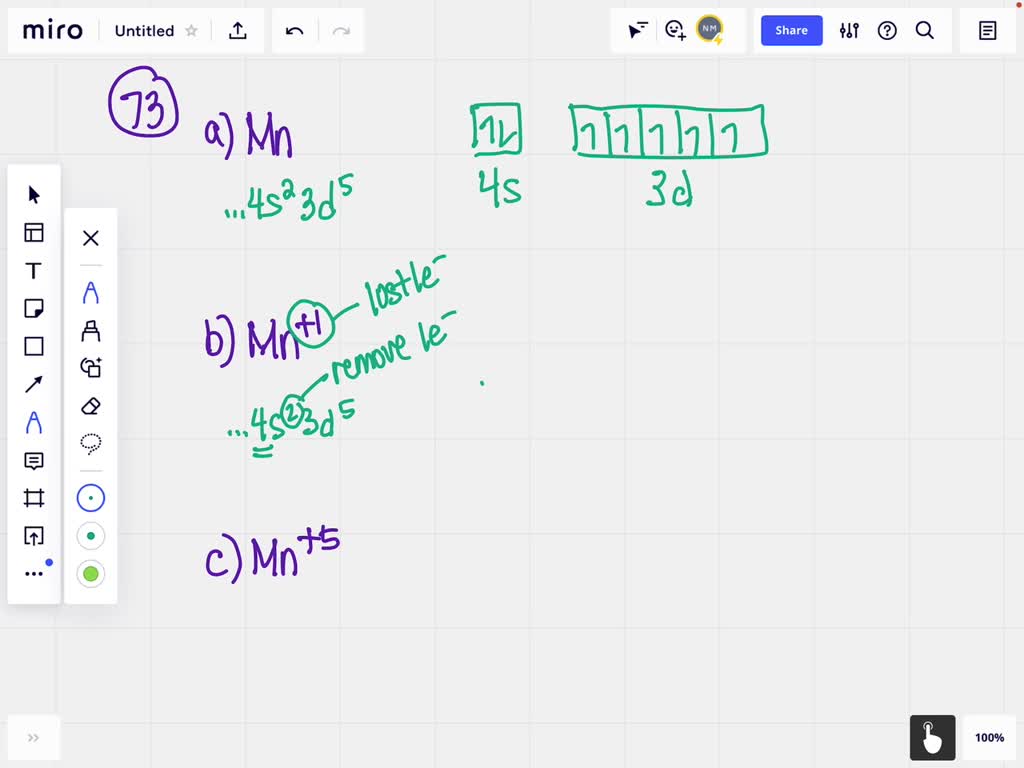
B) Fill in the orbital energy diagram for the manganese(II)ion. 3d 45 3p El 3s AV NN 2p AV 2s AV Is The lowest Elevels are already filled in for you. C) Fill in the orbital energy diagram for the fluoride ion. 2p E 2s 1s
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
Electron configuration of sodium atom through orbital diagram. Atomic energy levels are subdivided into sub-energy levels. These sub-energy levels are called orbital. The sub energy levels are expressed by 'l'. The value of 'l' is from 0 to (n - 1). The sub-energy levels are known as s, p, d, f.
The molecular, sp 3 orbitals are arranged in a tetrahedron, with bond angles of 109.5 o. Each of the 1s orbitals of H will overlap with one of these hybrid orbitals to give the predicted tetrahedral geometry and shape of methane, CH 4. Hybridization also changes the energy levels of the orbitals. The 2s orbital of carbon is lower in energy than the 2p orbitals, since it is more penetrating.
Answer to Solved Fill in the orbital energy diagram for the fluoride. ... Question: Fill in the orbital energy diagram for the fluoride ion. Ć 5 2p E 2s 1s ...
How to Write Electron Configurations. Step-by-step tutorial for writing the Electron Configurations.
Fill in the orbital energy diagram for the fluoride ion. 2p El 2s 1s 4 more group attempts remaining Submit Answer Retry Entire Group ; Question: Fill in the orbital energy diagram for the fluoride ion. 2p El 2s 1s 4 more group attempts remaining Submit Answer Retry Entire Group
Answer: This just shows energy levels so let's take this a step further. Atomic Electron Configurations And I'm not having any luck but if you go to this site, you should be about to see what the 1s, 2s, 2px, 2py, 2pz, and 3s orbitals look like together. Jmol orbital structures If not, see what...





0 Response to "38 fill in the orbital energy diagram for the fluoride ion."
Post a Comment