41 energy diagram transition state
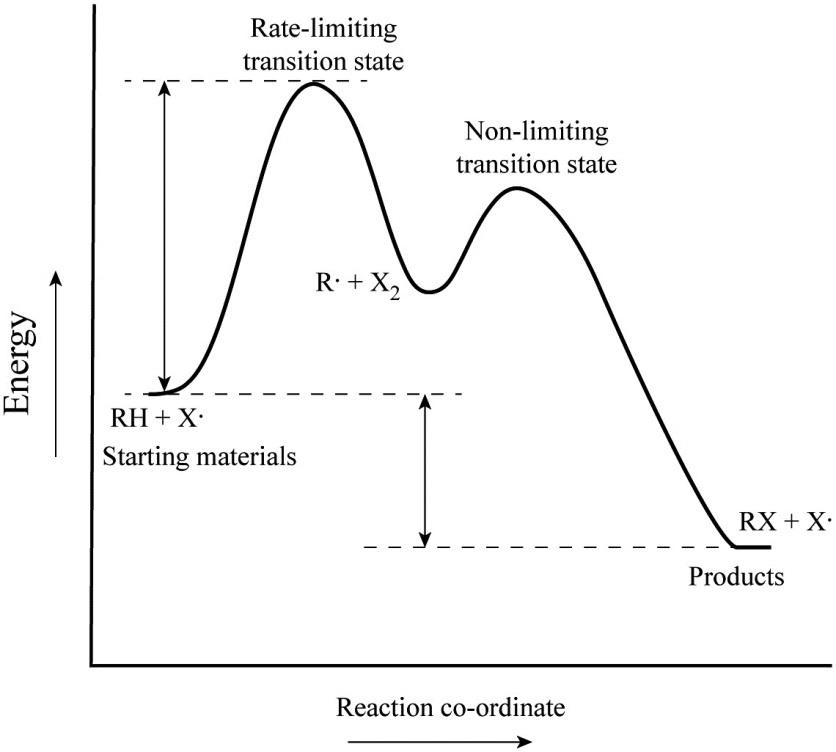
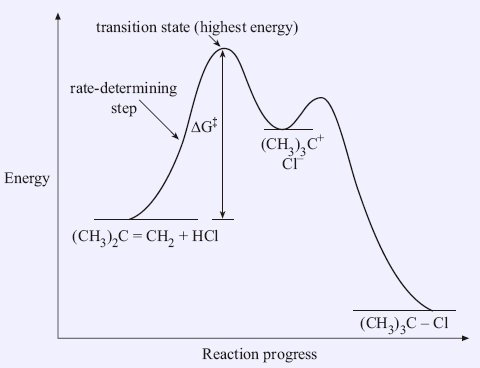
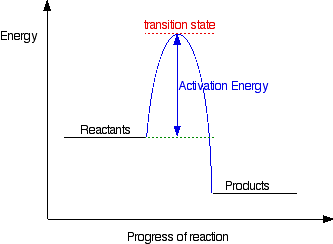
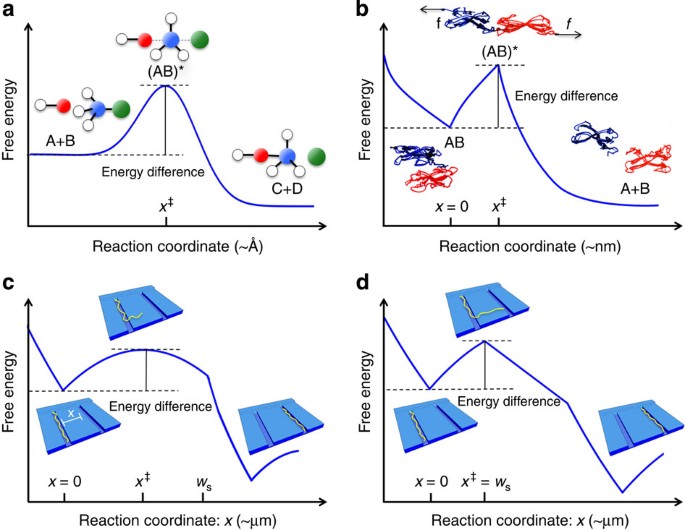
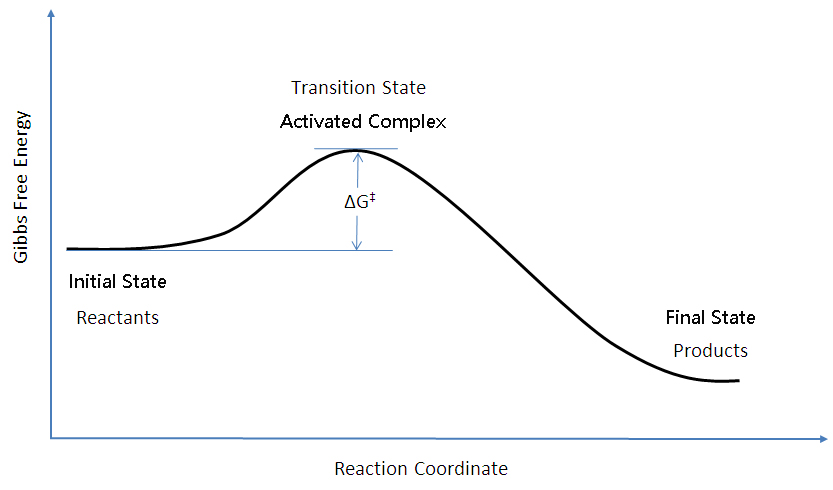
An energy diagram refers to the plot which shows the reactants’ relative potential energies, its transition states, as well as the products progression of the reaction with time. It is a plot between potential energy and reaction progress or time. It tells us about the reaction whether it is endothermic or exothermic. Energy Energy Diagrams 6 • Transition state ‡: – An unstable species of maximum energy formed during the course of a reaction. – A maximum on an energy diagram. • Activation Energy, ∆G‡: The difference in Gibbs free energy between reactants and a transition state. – If ∆G‡ is large, few collisions occur with sufficient
30 May 2020 — draw Reaction Energy Diagrams from the thermodynamic and kinetic data/information · use a Reaction Energy Diagram to discuss transition states, ...

Energy diagram transition state
The diagram for hydrogen is shown above. The n = 1 state is known as the ground state, while higher n states are known as excited states. If the electron in the atom makes a transition from a particular state to a lower state, it is losing energy. Reaction coordinate diagrams — Figure 1: Reaction Coordinate Diagram: Starting material or reactant A convert to product C via the transition state B. A potential energy diagram for this reaction shows the transition state (TS) as the highest point on the pathway from reactants to products. If you look carefully at the progress of the S N 2 reaction, you will realize something very important about the outcome.
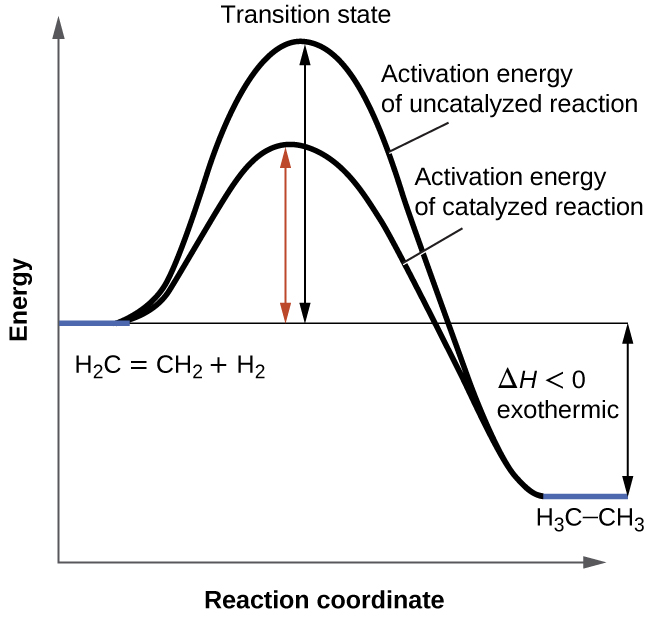
Energy diagram transition state. Transition state theory has been successful in calculating the standard enthalpy of activation, the standard entropy of activation, and the standard Gibbs energy of activation. Between products and reactants, there exists the transition state. The activated complex is a higher-energy, reactant-product hybrid. Energy Diagrams # Transition States vs. Intermediates! • A transition state occurs at an energy maxima. ! "The one on the left shows a Br- bond breaking and a Cl- bond forming." • Transition states cannot be isolated or directly observed.! • What might explain why transition states are so unstable?! Energy Diagrams # Transition States! 10 Jul 2016 — Figure 1-1 An energy diagram for the first step in the reaction of ethylene with HBr. The energy difference between reactants and the transition ... In a multi-step reaction, each step has a transition state and corresponding activation energy. The transition states of such reactions are punctuated with ...
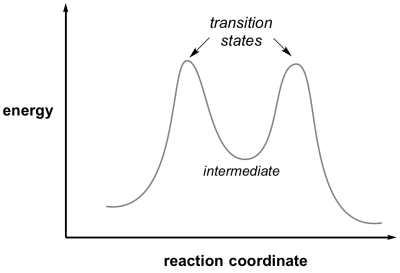
Transition state is the highest point (or points) on the reaction coordinate diagram. Those are the “peaks” or the “hills” in the picture. A more strict ... the beginning state. The energy of the TS≠ must be closer to the beginning state. There’s just no other way to draw the diagram. We call this TS≠ “early” since the structure of the transition state has not evolved far from its starting point. Case 2: the ending state is higher than the beginning state – In this case The peak of this energy diagram represents the transition state: an intermediate stage in the reaction from which the reaction can go in either direction. Reactions with a high activation energy will proceed very slowly, because only a few molecules will obtain enough energy to reach the transition state – even if they are highly exergonic. Transition state theory (TST), also called activated complex theory, is often introduced in general chemistry courses when discussing kinetics. A reaction energy diagram is used to follow the progress of the reaction from reactants through a transition state to products (see figure 1). The reaction energy diagram plots the
Chemistry. Chemistry questions and answers. 49. In an energy diagram, the transition state establishes whether the reaction releases or produces energy. a. True b. False 50. From the following compound, [CH3CH2CH (Br) CH2CH3] we can say that: a. It exists as a pair of enantiomers. A potential energy diagram for this reaction shows the transition state (TS) as the highest point on the pathway from reactants to products. If you look carefully at the progress of the S N 2 reaction, you will realize something very important about the outcome. Reaction coordinate diagrams — Figure 1: Reaction Coordinate Diagram: Starting material or reactant A convert to product C via the transition state B. The diagram for hydrogen is shown above. The n = 1 state is known as the ground state, while higher n states are known as excited states. If the electron in the atom makes a transition from a particular state to a lower state, it is losing energy.
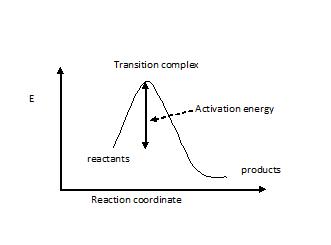
Difference Between Activated Complex And Transition State Compare The Difference Between Similar Terms
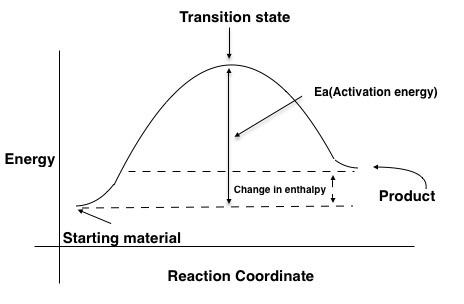
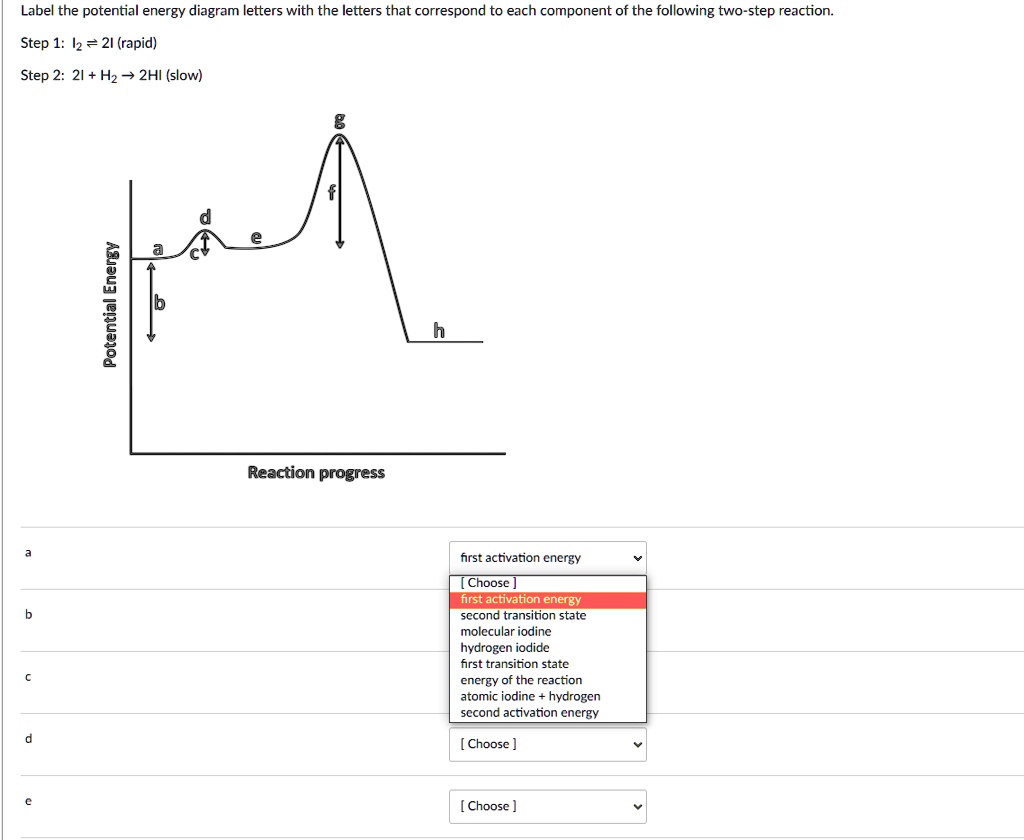
Draw An Energy Diagram For A Reaction In Which The Products Are Higher In Energy Than The Starting Materials And E A Is Large Clearly Label All Of The Following On The Diagram

3 Consider The Reaction Energy Diagrams Below Indicate All Statements That Are True Transition State Transition Homeworklib



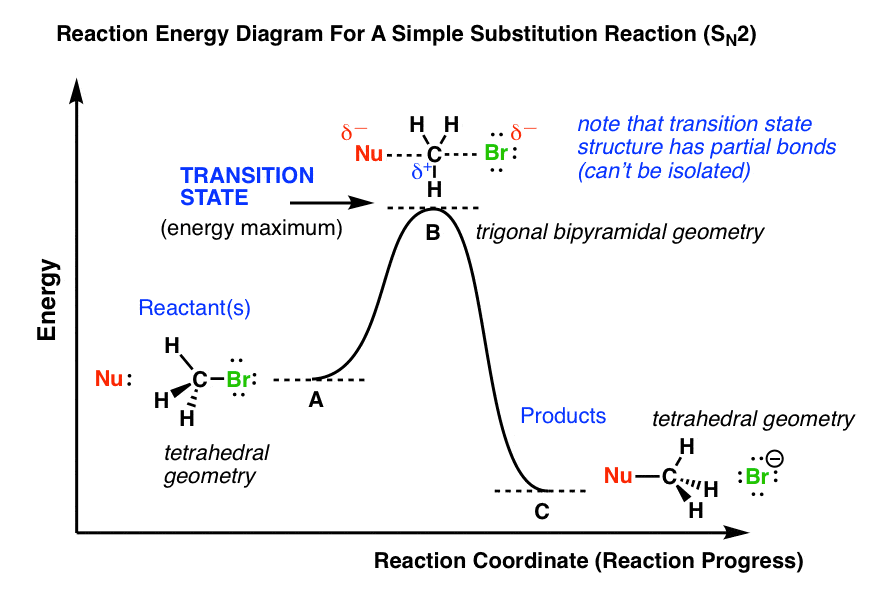

















0 Response to "41 energy diagram transition state"
Post a Comment