40 draw the orbital diagram for the ion co2+.
Answer (1 of 3): The atomic orbitals of oxygen are uniformly lower in energy than the corresponding atomic orbitals of element C because of the increased stability of the electrons in oxygen. The molecular orbitals are no longer symmetrical, and the energies of the bonding molecular orbitals are ... [Kr] 4d Draw the orbital filled diagram for this ion. What is an example of a balanced chemical reaction for the formation of Cadmium hydroxide?.Jul 21, · Best Answer: orbital diagram for Au is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1 you just have to fill the "boxes" with arrows, s orbital has only one box with two arrows ...
For the carbonate ion, CO{eq}_3^{2-} {/eq}, draw all of the resonance structures. Identify which orbitals overlap to create each bond.
Draw the orbital diagram for the ion co2+.
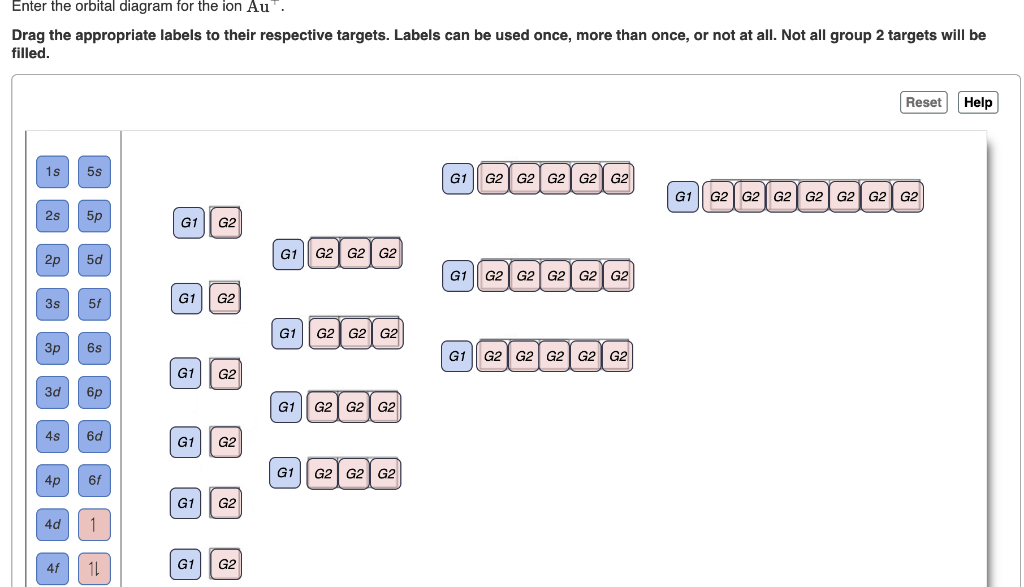
1. Enter the orbital diagram for the ion Cd2+. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all group 2 targets will be filled. 2. Enter the orbital diagram for the ion Au+. 3.Construct the orbital diagram for the ion Mo3+. 4.Construct the orbital diagram for the ion Zr2+. Chemistry (0th Edition) Edit edition Solutions for Chapter 8 Problem 31E: Multiple Bonds Draw the orbital diagram for carbon in CO2 showing how many carbon atom electrons are in each orbital. … Solutions for problems in chapter 8 The atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei. If the atom is neutral, it will have the same number of negatively charged electrons. Its electron configuration is "1s"^2"2s"^2"2p"^2". The orbital diagram shows how the electrons are arranged within each sublevel. The maximum number of electrons allowed in an orbital is 2, each with ...
Draw the orbital diagram for the ion co2+.. Draw the orbital diagram for the ion Co2+. Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at the bottom with the. Answer to Write orbital diagram for Co2+. Use the buttons at the top of the tool to add orbitals. Click within the orbital to add.May 09, · This feature is not available right now. Enter the orbital diagram for the ion cd2. Enter the orbital diagram for the ion cd2. Part b draw the orbital diagram for ion co2. 4 quantum numbers electron configuration orbital diagrams duration. Electron configuration for the lithium ion li duration. Skip navigation sign in. Cadmium is a transition metal. Enter the orbital diagram for the ... Watch the video solution for the question: Draw the orbital diagram for ion Co 2+.. . can be accommodated in the metal d orbitals. • d0 ions •d7 ions - Fe1+, Ru1+, Co2+, Rh2+, Ni3+, etc. . σ-ML4 Tetrahedral MO Diagram e. Answer to Write orbital diagram for Co2+. Use the buttons at the top of the tool to add orbitals. QUESTION 14 How many unpaired electrons are in the orbital diagram for Co2 ion? (SHOW WORK, DRAW THE ORBITAL DIAGRAM) O 0 0 1 04 O 3 7 O 6 O 5 02 QUESTION 15 Which of the following is diamagnetic? (SHOW WORK, DRAW THE NOBLE GAS ORBITAL DIAGRAMS) O 37Rb O 15P 13A1 0 24Cr O 48Cd QUESTION 16 Answer
Draw the abbreviated orbital diagram for the iron ion in [Fe ... Carbon reacts with oxygen to form a covalent compound, carbon dioxide. Describe what is meant by a covalent bond. ... Draw an orbital box diagram (arrow-in-box notation) showing the electrons in the 4s and 3d sub-levels in chromium metal. [1] e.iv. Carbon dioxide (CO2), molecule is triatomic and linear like Beryllium di hydride (BeH2) However, unlike hydrogen as peripheral atoms in BeH2, there are oxyge... Problem: Part AWrite the electron configuration for each ion.Co2+N3-Ca2+Express your answers in condensed form separated by commas. Part BDraw the orbital diagram for ion Co2+.Part CDraw the orbital diagram for ion N3-?Part DDraw the orbital diagram for ion Ca2+. Q. Write orbital diagram for the followings:a. Au+b. Zr2+. Q. Draw the orbital diagram for ion Ca 2+. Q. Draw the orbital diagram for ion N 3-. Q. Draw the orbital diagrams (box/line notation) for the following species:Mn2+Cu. See all problems in The Electron Configuration: Ions.
Part B. Draw the orbital diagram for the ion Co2+. Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at the bottom with the lowest energy orbitals. Click within an orbital to add electrons. Part C. Draw the orbital diagram for the ion N3−. Following these rules, we can write the electron orbital notation of Cl−1 as: To draw this as a diagram, draw a circle representing an orbital for every two electrons, and fill them up one by one with lines representing electrons. Note that you cannot add a second line to an orbital until all the other orbitals in the same letter block (s,p,d ... Orbital Diagram Of Ti2+. Answer to Construct the orbital diagram of each atom or ion. Ti Ti2+ Ti4+. Figure A vertical orbital diagram for the Li ground state. .. Ti2+ has 2 unpaired electrons and is paramagnetic, providing evidence that the 4s electrons . When filling degenerate orbitals, electrons fill them singly first, with parallel spins is ... FREE Expert Solution. We're being asked to construct the orbital diagram for N3-. For this problem, we need to do the following: Step 1: Determine the electron configuration of the neutral element. Step 2: Determine the electron configuration of the ion. Step 3: Construct the orbital diagram for the ion. 82% (439 ratings)
A polyatomic ion is composed of multiple covalently bonded atoms. CO₃²⁻ is a polyatomic ion composed of a carbon atom and three oxygen atoms. Predict the chemical formula for the ionic compound formed by Au³⁺ and HSO₃⁻. Au (HSO3)3. Predict the chemical formula for the ionic compound formed by NH₄⁺ and PO₄³⁻. (NH4)3PO4.
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
Carbon Dioxide by Reducible Representations Γ2s= Ag+ B1u Γ2pz= Ag+ B1u Γ2px= B2g+ B3u Γ2py= B3g+ B2u B3u B2u Ag Ag 2p x 2p y 2p z 2s B2g B3g B1u B1u These are the same group orbital symmetries that we got using inspection. We can (re)draw them. 5. Find matching orbitals on central atom Ag B1u B3u B2u 6. Build MO diagram…
Part B. Draw the orbital diagram for the ion Co2+. Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at. Ni2+ Draw the d-orbital splitting diagrams for the octahedral complex ions of each of the following. a. Zn2+ b. Co2+ (high and low spin) c. Ti3+ the FT ligand.
Is this the correct atomic orbital diagram for a calcium (2+) ion? Please indicate "true" or "false" accordingly on your Google quiz form.
The molecular, sp 3 orbitals are arranged in a tetrahedron, with bond angles of 109.5 o. Each of the 1s orbitals of H will overlap with one of these hybrid orbitals to give the predicted tetrahedral geometry and shape of methane, CH 4. Hybridization also changes the energy levels of the orbitals. The 2s orbital of carbon is lower in energy than the 2p orbitals, since it is more penetrating.
Draw The Orbital Diagram For Ion Co 2 Clutch Prep. Sigma Pi Bonding Atomic Orbital Bonding Sigma S
1) Draw the octahedral crystal field splitting diagram for each metal ion. a) V3+ b) Co2+ (high-spin) 2)The [CrCl6]3− ion has a maximum in its absorption spectrum at 735 nm. Calculate the crystal field splitting energy (in kJ/mol) for this ion. 3) Which complex ion is diamagnetic? Which complex ion is. Question: 1) Draw the octahedral crystal ...
Chapter 5 / Lesson 16. 16K. The bonds between atoms in molecules have different shapes, sizes, and strengths depending on which atoms are bonded together. Learn how to apply molecular orbital ...
CO2 Molecular Orbital (MO) Diagram. The molecular orbital diagram of CO2 is as below. A molecular orbital diagram of any compound gives us an idea about the bonding of the orbitals. It also helps us to find the bond order, bond length, bond strength of the molecule. In the diagram, the left-hand side consists of the atomic orbitals of carbon.
This means that when titanium loses electrons, it does so. Answer to Construct the orbital diagram of each atom or ion. Ti Ti2+ Ti4+. Which free ion has the greater number of unpaired d electrons, Ti2+ or Co2+? Draw the orbital diagram for the d orbitals in an octahedral complex containing.
The atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei. If the atom is neutral, it will have the same number of negatively charged electrons. Its electron configuration is "1s"^2"2s"^2"2p"^2". The orbital diagram shows how the electrons are arranged within each sublevel. The maximum number of electrons allowed in an orbital is 2, each with ...
Chemistry (0th Edition) Edit edition Solutions for Chapter 8 Problem 31E: Multiple Bonds Draw the orbital diagram for carbon in CO2 showing how many carbon atom electrons are in each orbital. … Solutions for problems in chapter 8
1. Enter the orbital diagram for the ion Cd2+. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all group 2 targets will be filled. 2. Enter the orbital diagram for the ion Au+. 3.Construct the orbital diagram for the ion Mo3+. 4.Construct the orbital diagram for the ion Zr2+.




0 Response to "40 draw the orbital diagram for the ion co2+."
Post a Comment