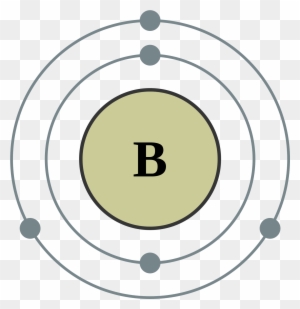
39 orbital filling diagram for boron
Electron configurations are a shorthand form of an orbital diagram, describing which orbitals are occupied for a given element. For example, 1s2 2s2 2p1 is the electron configuration of boron. Use this tool to generate the electron configuration of arsenic (As). An orbital diagram is similar What is the orbital diagram for. For example, write the electron configuration of scandium, Sc: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 1. So for scandium the 1 st and 2 nd electron must be in 1s orbital, the 3 rd and 4 th in the 2s, the 5 th through 10 th in the 2p orbitals, etc. 6/14/ Ch 8 4/18 Correct Part B Complete ...
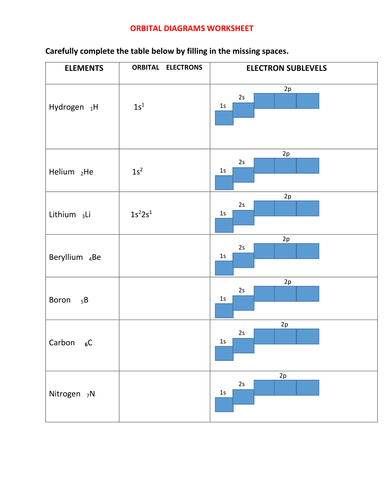
1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. Table: Element Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium
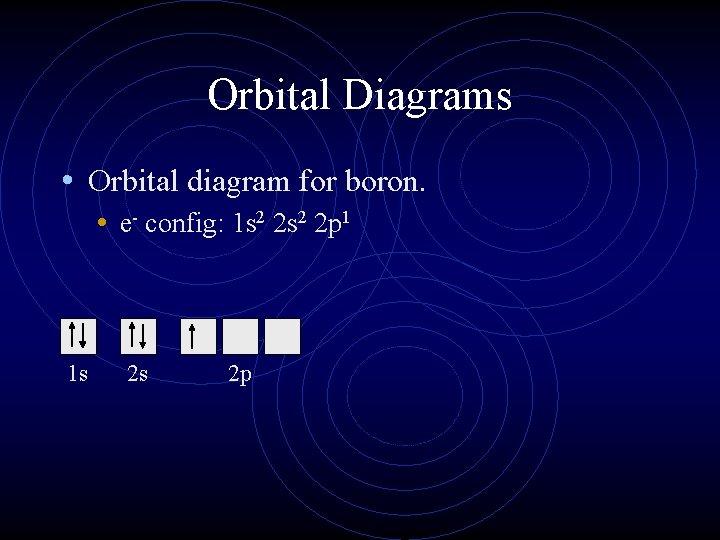
Orbital filling diagram for boron
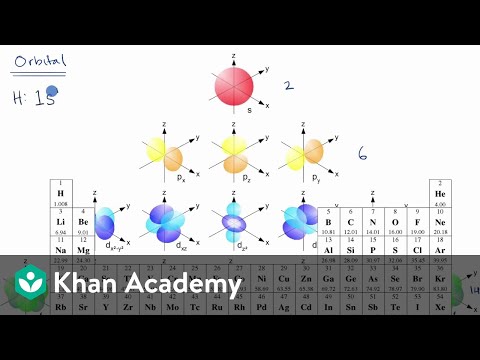
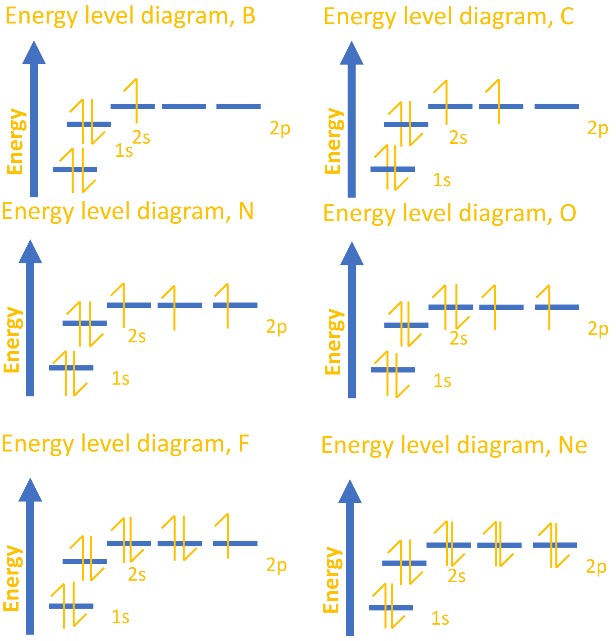
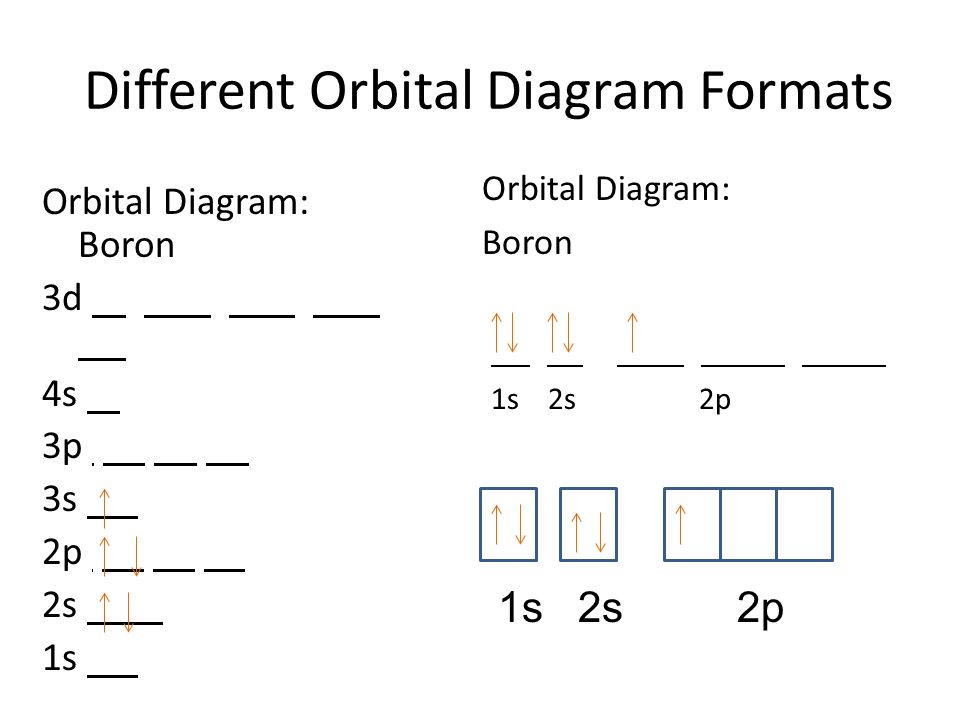
Orbital filling diagrams essentially just turn this big list of electron locations . In the same way, the orbital filling diagram for nitrogen will be. Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s. The orbital notation for boron is shown. Note that the first arrow of each orbital is up-facing. Remember, according to Hund's Rule, each of the 2p orbitals will receive one electron (up-facing arrow) before any will get a second electron (down-facing arrow). The total number of arrows in the notation will equal the atomic number. Boron (B) has an atomic mass of 5. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.
Orbital filling diagram for boron. Orbital Diagram, electron configuration, and the noble gas notation for a silicon (Si) atom. The orbital filling diagram of boron. I skipped past beryllium beca use I was getting bored. The electron configuration of boron is 1s²2s²2p¹, which means that the re are two electron s in the 1s orbital, two electron s in the 2s orbital, and one electron in the 2p orbitals. This gives us an orbital filling diagram of: QUESTION 37 YOU MUST SUBMIT WORK FOR THIS PROBLEM INTO A SEPARATE ASSIGNMENT. Write the condensed orbital filling diagram of the following: A. iron (Fe) B. boron (B) Use the similar scheme as showed for carbon (C): [He] 111 11 | 1 | || 2s 2p 1. use the special signs - click on the 2 icon 2. switch to Arrows panel - on the bottom of the list 3. select appropriate arrows 4. make at least 5 ... Diagram of Hund's rule in boron, carbon, nitrogen, and oxygen. Figure 1. The 2p.Show transcribed image text Show the orbital-filling diagram for N. Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital.
Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of; Question: Draw an orbital diagram for boron. Use this tool to draw the orbital diagram. Draw an orbital diagram for scandium (Sc). Use this tool to draw the orbital diagram. How many orbitals are there in the third shell (n = 3)? Express your answer numerically ... For example, 1s22s22p1 is the electron configuration of boron. Use this tool to generate the electron configuration of arsenic (As). Question: Part C Electron configurations are a shorthand form of an orbital diagram, describing which orbitals are occupied for a given element. For example, 1s22s22p1 is the electron configuration of boron. The next element is boron with 5 electrons. The orbital diagram for boron as shown has the one electron in the 2p orbital. The electron can be placed in any of the three 2p orbitals. The electron configuration for boron is 1s 2 2s 2 2p 1. This preview shows page 2 - 3 out of 3 pages. Table: Element Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium.
You can complete the orbital diagrams of Boron (B) and Scandium (Sc) by referring to the periodic table, locating the position of each element in it, and ...Sep 16, 2020 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. Table: Element Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium Orbital Diagram of All Elements Diagrams; 1: Orbital diagram of Hydrogen (H) 2: Orbital diagram of Helium (He) 3: Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine ... Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Use the buttons at the top of the tool to add %(8). Question: Orbital Diagrams Draw an orbital diagram for boron. Use this tool to draw the orbital diagram.
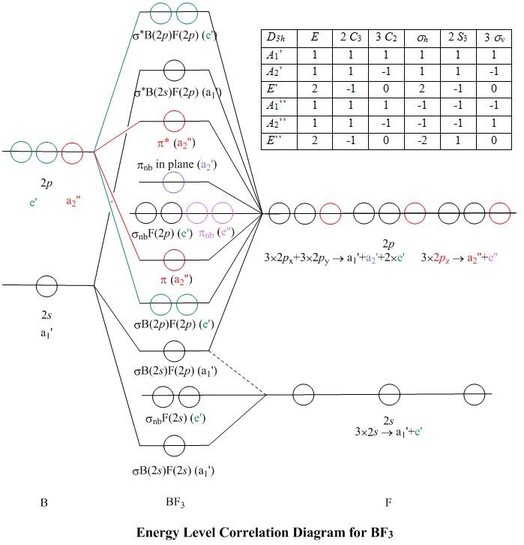
Draw And Explain The M O Diagram Of Boron Molecule Sarthaks Econnect Largest Online Education Community
May 18, 2021 — An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom.

Draw The Molecular Orbital Diagram For I Be2 Ii B2 And Predict Bond Order And Magnetic Properties From Chemistry Chemical Bonding And Molecular Structure Class 11 Haryana Board English Medium
Use this tool to draw the orbital diagram. Draw an orbital diagram for scandium (Sc). From the orbital diagram, we can write the electron configuration in an abbreviated When we reach boron, with Z = 5 and five electrons, we must place the fifth . After filling the first five rows, we still have 80 − 54 = 26 more.Orbital Filling Diagrams.
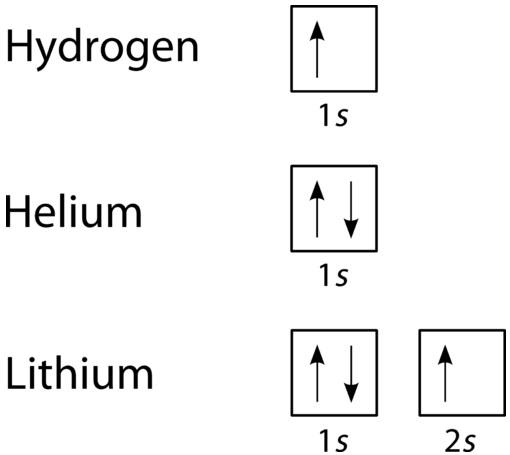
Thus, the electron configuration and orbital diagram of lithium are: An atom of the alkaline earth metal beryllium, with an atomic number of 4, contains four protons in the nucleus and four electrons surrounding the nucleus. The fourth electron fills the remaining space in the 2s orbital. An atom of boron (atomic number 5) contains five electrons.
1. Orbital Filling Diagram 02 Ex. 2, Electron Configuration 02 Ex. (gives the most information) Is (quicker to draw than orbital filling diagrams) Dot Pb 3. Electron Dot shows only the valence (outer energy level) electrons Oxygen atom Ex. 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following ...
The orbital filling diagram of boron. I skipped past beryllium because I was getting bored. The electron configuration of boron is 1s²2s²2p¹, which means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbitals. This gives us an orbital filling diagram of.
An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the ...
Boron is the fifth element with a total of 5 electrons. The electron configuration of boron is 1s²2s²2p¹ which means that there are two electrons in the 1s orbital two electrons in the 2s orbital and one electron in the 2p orbitals. This gives us an orbital filling diagram of. The remaining electron will go in the 2p orbital.
Orbitals are filled from lowest energy to ... Orbital Diagrams. • Shows the distribution of electrons within orbitals ... Orbital Diagram for Boron.24 pages
(quicker to draw than orbital filling diagrams) 1 s2 2s2 2p4 3. Electron Dot shows only the valence (outer energy level) electrons EX. Oxygen atom 1 . Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. LKrl 10 Table. a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g ...
The orbital filling diagram of boron. I skipped past beryllium because I was getting bored. The electron configuration of boron is 1s²2s²2p¹, which means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbitals. This gives us an orbital filling diagram of:
Arrange the following in order of decreasing stability. a blank molecular orbital diagram (part a 1 figure) has been provided to you. rank the fluorine species from most to least stable. to rank items as equivalent, overlap them. f2, f2+, f2-
Boron is the fifth element with a total of 5 electrons. In writing the electron configuration for Boron the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for B goes in the 2s orbital. The remaining electron will go in the 2p orbital.

Electron Configuration The Electronic Structure Of Atoms Atomic Orbital Electron Configuration Of Boron Angle Text Png Pngegg
Complete an orbital diagram for boron. Boron is the fifth element with a total of 5 electrons. Use this tool to draw the orbital diagram. Therefore the b electron configuration will be 1s22s22p1. Lower energy subshells fill before higher energy subshells. Use the buttons at the top of the tool to add orbitals.
Draw an orbital diagram for boron. 2. Draw an orbital diagram for scandium (Sc). 3. How many orbitals are there in the third shell (n = 3)? 4. Show the orbital-filling diagram for N (nitrogen ...

Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes
Here is an example of orbital configuration for Hydrogen, Helium and Carbon. The simplest atom hydrogen has 1 electron. It will go into the 1s orbital with a spin in either direction. We can represent this with either an orbital filling diagram or an electron configuration. The next atom is helium. It has 2 electrons.
Boron has:- 1s2 2s2 2p1. Why are the outermost electrons the only ones included in the orbital filling diagram and the electron dot diagram?

There Are Three Ways To Indicate The Arrangement Of Electrons Around An Atom 1 Orbital Filling Diagram Gives The Most Information Ay Ex O2 1s 2s 2p 2
Boron (B) has an atomic mass of 5. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.
The orbital notation for boron is shown. Note that the first arrow of each orbital is up-facing. Remember, according to Hund's Rule, each of the 2p orbitals will receive one electron (up-facing arrow) before any will get a second electron (down-facing arrow). The total number of arrows in the notation will equal the atomic number.
Orbital filling diagrams essentially just turn this big list of electron locations . In the same way, the orbital filling diagram for nitrogen will be. Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s.

Electron Arrangement Practice Example 1 Docx Name Electron Arrangements There Are Three Ways To Indicate The Arrangement Of Electrons Around An Atom Course Hero















0 Response to "39 orbital filling diagram for boron"
Post a Comment