39 orbital diagram for argon
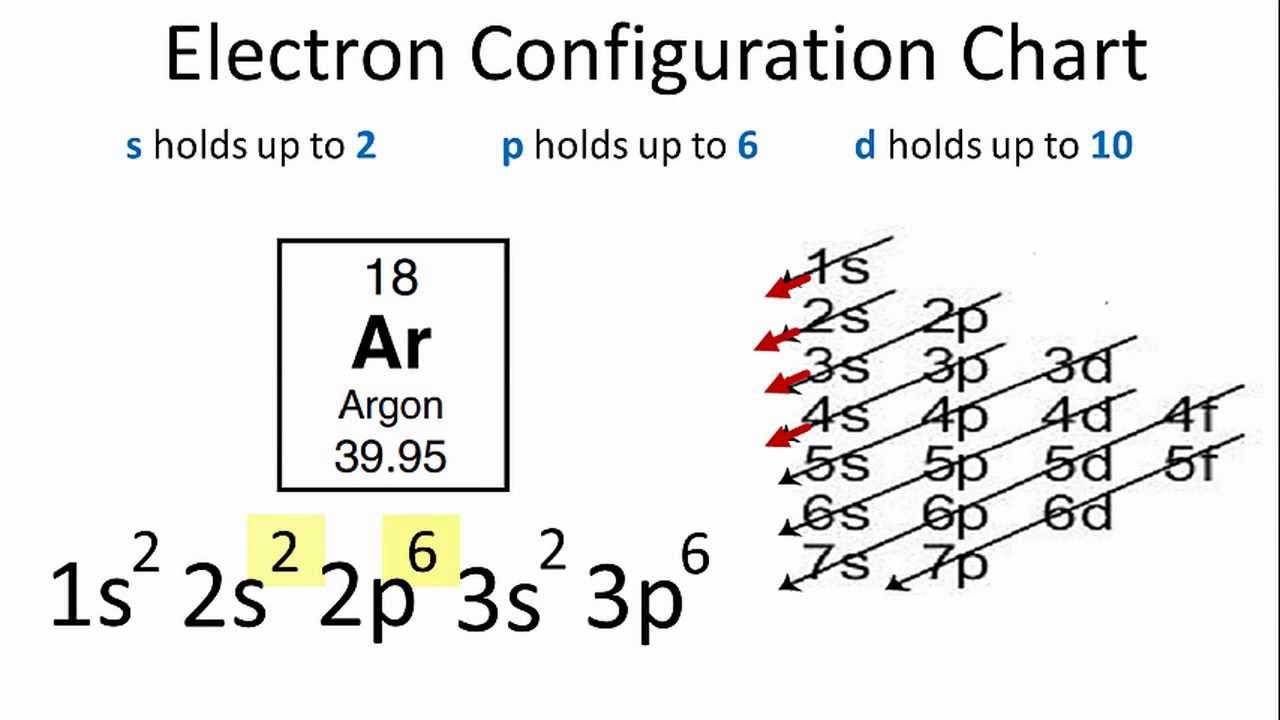
24 Jan 2021 — Argon's electron configuration for the first two electrons goes in the 1s orbital. 1s only hold two electrons and the next 2 electrons for Argon ... Berdasarkan jenis orbital yang ditempati oleh elektron terakhir, unsur-unsur dalam sistem periodik dibagi atas blok s, blok p, blok d, dan blok f. Blok s: golongan IA dan IIA; Blok s tergolong logam aktif, kecuali H dan He. H tergolong nonlogam, sedangkan He tergolong gas mulia. Blok p: golongan IIIA sampai dengan VIIIA
In this video we will write the electron configuration for Br-, the Bromide ion. We'll also look at why Bromine forms a 1- ion and how the electron configuration for Br- is the same as the Nobel gas Argon. To begin with, Bromine (Br) has an electronic configuration of 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5.
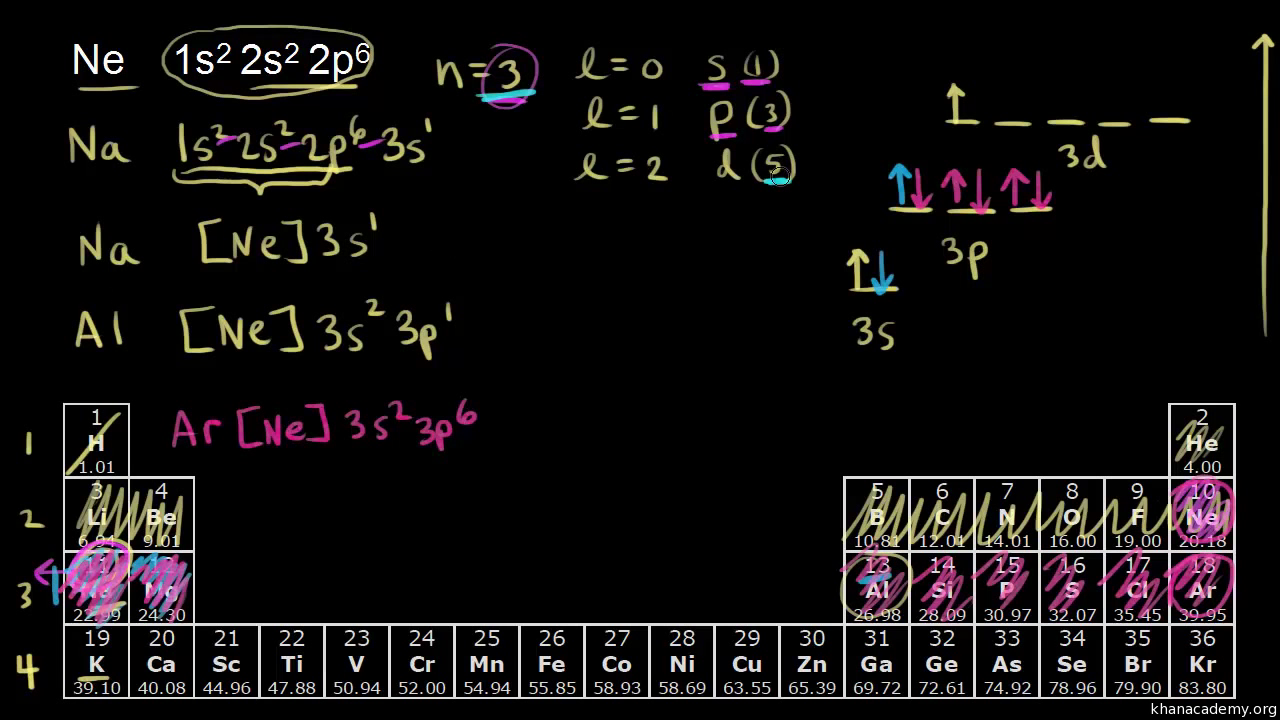
Orbital diagram for argon
An orbital defines a region within an energy level where there is a high probability of finding a pair of electrons. There can be a maximum of two electrons in each orbital. This is why the electrons are often shown in pairs within an energy level. Tell students that the rows across on the periodic table are called periods. Period 1 Hydrogen In writing the electron configuration for Argon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons ...18 Nov 2013 · Uploaded by Wayne Breslyn Unconventional superconductivity in non-centrosymmetric superconductors has attracted a considerable amount of attention. While several lanthanide-based materials have been reported previously ...
Orbital diagram for argon. Original prompt: [[WP] Bryan Wetherspoon, 26, an interstellar pilot, has just been made redundant owing to increased automation. Vengeful, he is now enroute to Persephone in 40 Eridani B to blow up Jupiter Command, the central Hive Mind of all AI systems across the human interstellar community.](https://www.reddit.com/r/WritingPrompts/comments/oxo78o/wp_bryan_wetherspoon_26_an_interstellar_pilot_has/) The talking head continued screaming, broadcasting his fury across *NeuroNet*. "Even...eve... The full electron configuration of Potassium (K) is 1s22s22p63s23p64s1. The abbreviated form - [Ar]4s1 - means the electron configuration of Argon (Ar), plus ... Earlier I was listening to [a podcast about neutrinos](http://www.bbc.co.uk/programmes/b0106tjc), and at one point someone mentions looking for argon atoms amongst [...a different kind, let's say helium for simplicity's sake] atoms as proof of something. I remember those diagrams in my text books, with the little nucleus and the orbiting electrons, but obviously that's not what you see under a scanning idontknowtheterm microscope or whatever the technology is. What do you see when you look at an... The correlation between CO 2 adsorption energy and the p orbital center of Si atoms is also observed for these endohedrally doped Si clusters, i.e., the smaller-size Si cluster has a higher p ...
The orbital diagram for germanium is. The orbital filling diagram for helium The electron configuration for helium is 1s². The symbol of lead is Pb lead has an Atomic Number of 82. If we gave you brief information then the first two electrons lie in the 1s orbital following the next 2 electrons it comes under the 2s orbital. The sequence of orbitals for electron configuration can be seen from this diagram. The maximum number of electrons that can be filled in each orbital are: s = 2.1 answer · Top answer: Hey there! We have to draw the electron configuration for a neutron atom of argon.The electron configuration of an atom or molecule is the distribution ... Oct 11, 2021 · Each box represents one orbital, and an orbital can only have a maximum number of two electrons. There are three 'p' orbitals, and on the left side, it is shown how the 'p' orbitals are filled. Argon (Ar) has an atomic mass of 18. ... Electron Configuration, [Ne] 3s2 3p6. 1s2 2s2 2p6 3s2 3p6. Orbital Diagram ... Lewis Dot Diagram of Argon (Ar).
23 Feb 2012 — Convert from orbital representation diagrams to electron ... Now, let's draw the electron configuration for Argon and place the electron ... Unconventional superconductivity in non-centrosymmetric superconductors has attracted a considerable amount of attention. While several lanthanide-based materials have been reported previously ... In writing the electron configuration for Argon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons ...18 Nov 2013 · Uploaded by Wayne Breslyn An orbital defines a region within an energy level where there is a high probability of finding a pair of electrons. There can be a maximum of two electrons in each orbital. This is why the electrons are often shown in pairs within an energy level. Tell students that the rows across on the periodic table are called periods. Period 1 Hydrogen
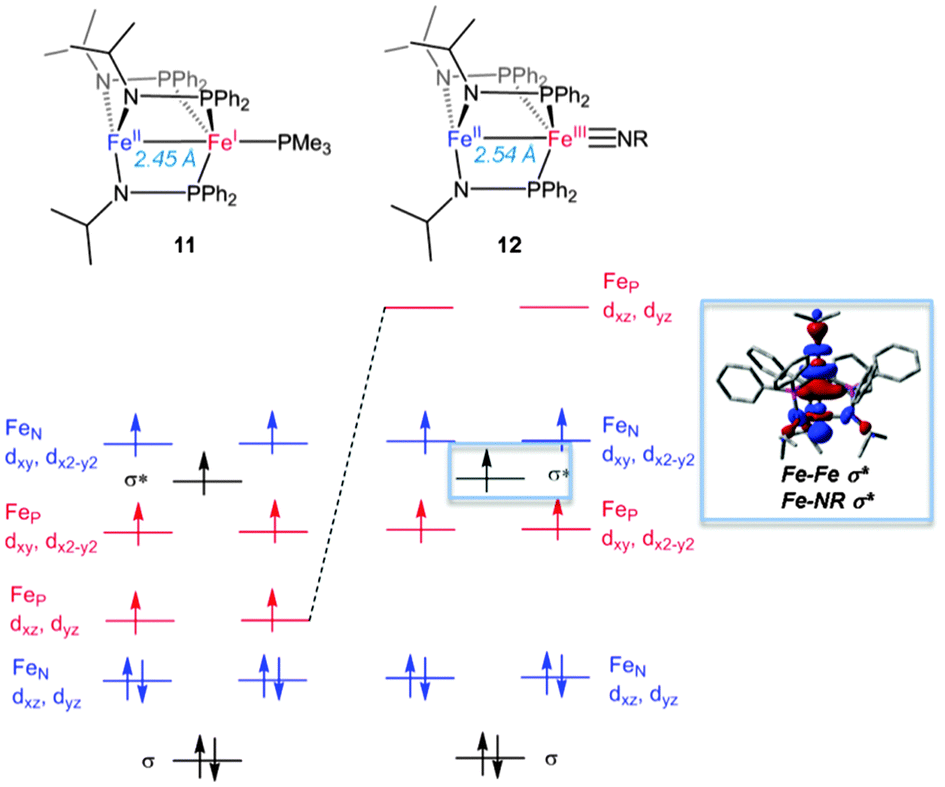
Metal Metal Multiple Bonding In C3 Symmetric Bimetallic Complexes Of The First Row Transition Metals Chemical Communications Rsc Publishing
Electron Configuration Argon Atom Electron Shell Png 600x600px Electron Configuration Area Argon Atom Atomic Number Download

18 Ar Argon Electron Shell Structure Schoolmykids Periodic Table Of The Elements Electron Configuration Atomic Structure
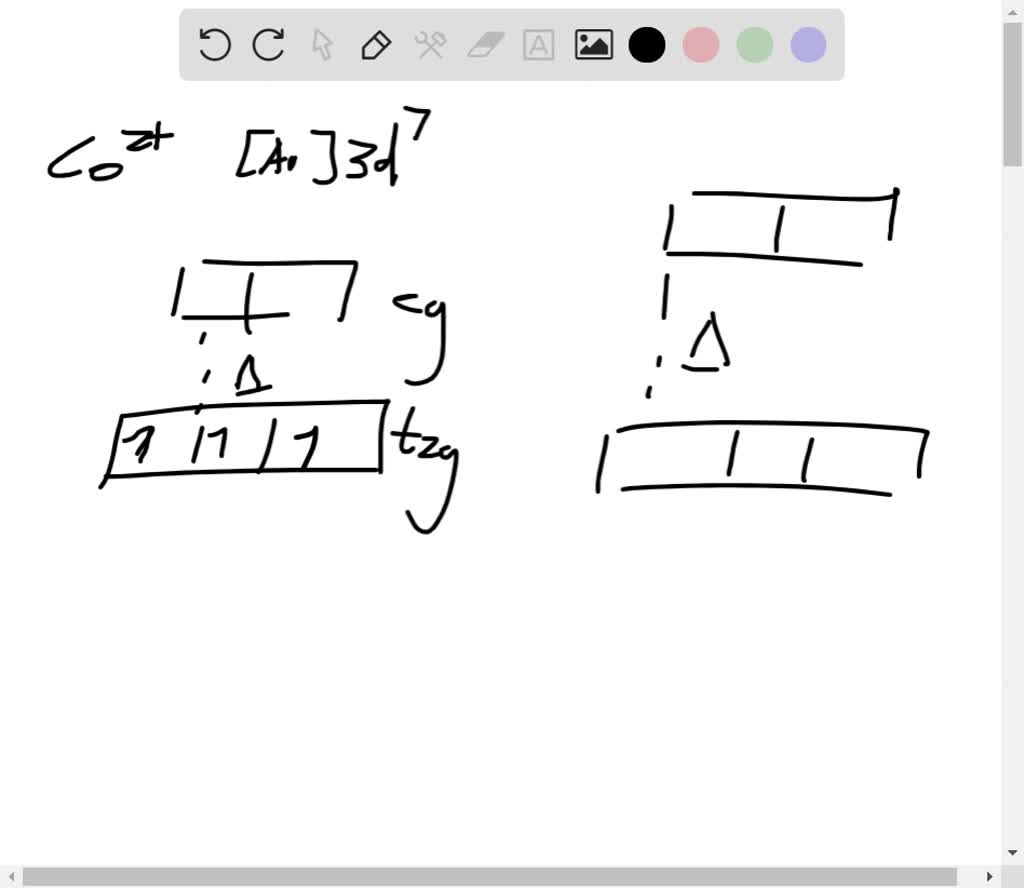
Solved The Complex Fecl6 3 Is More Paramagnetic Than Fe Cn 6 3 Explain In Terms Of Crystal Field Theory And Electron Configuration Show Orbital Diagram To Support Your Argument

Aufbau Principle Molecular Orbital Diagram Electron Configuration Bromine Atomic Orbital Others Png Pngwing

Orbital Diagram Argon Quantum Theory Hund S Rule Aufbau Principle And Pauli Exclusion Principle Youtube
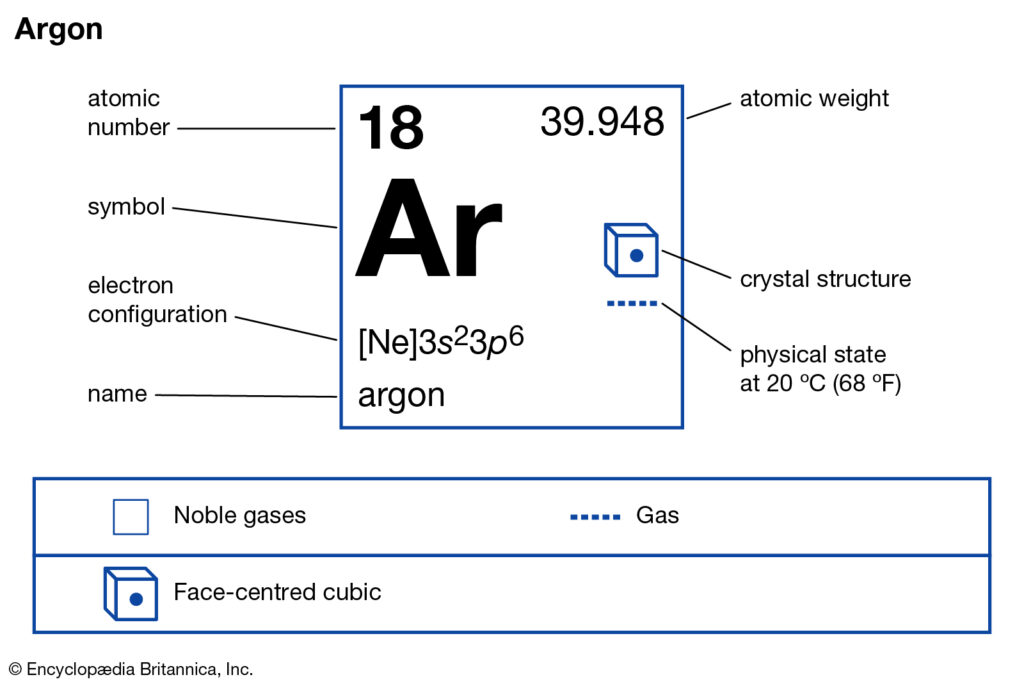
How Many Valence Electrons Does Argon Have Archives Dynamic Periodic Table Of Elements And Chemistry








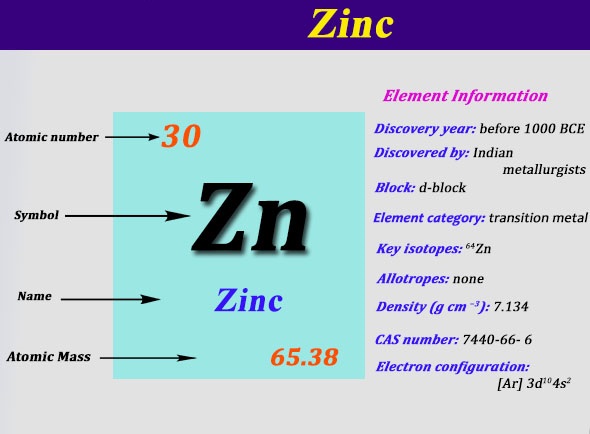




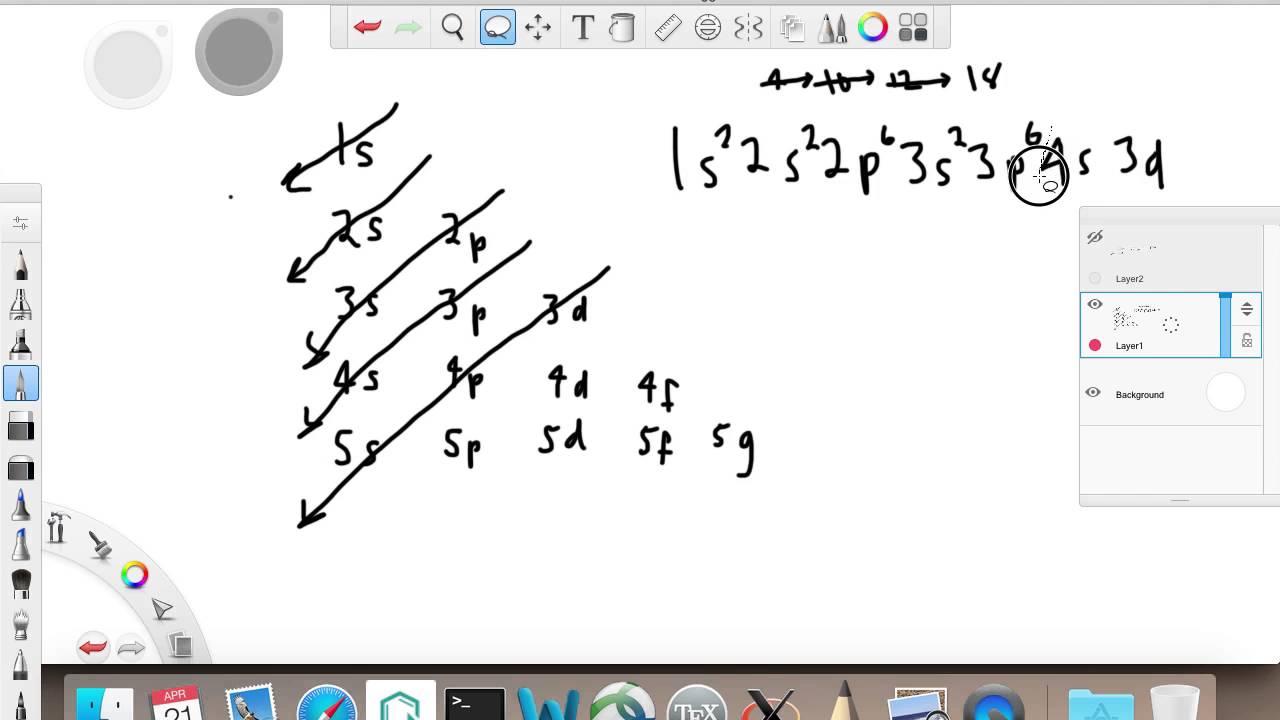
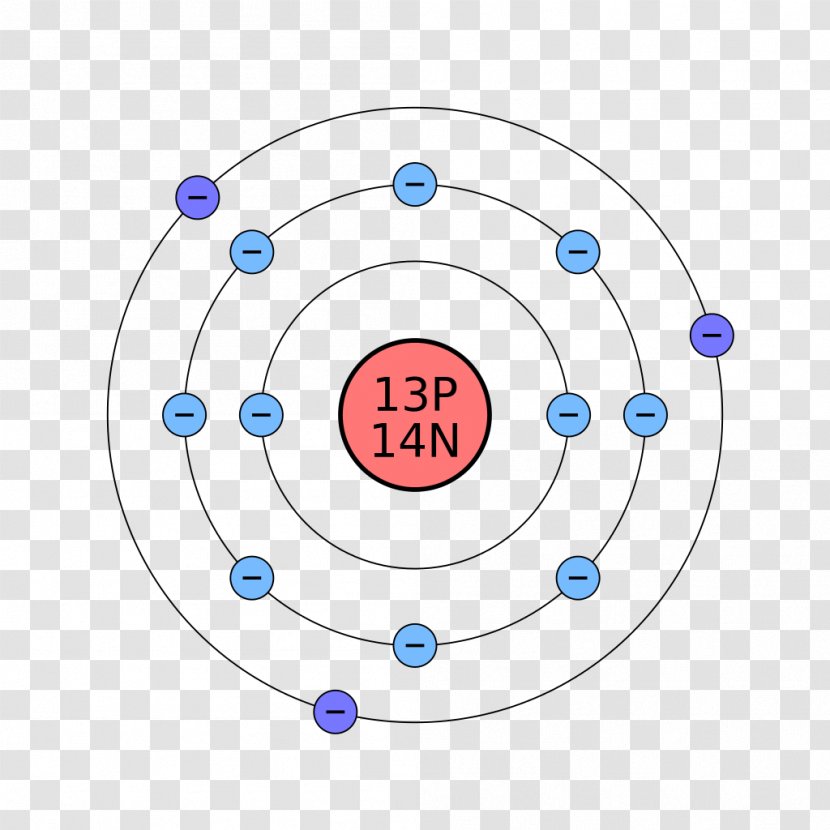












0 Response to "39 orbital diagram for argon"
Post a Comment