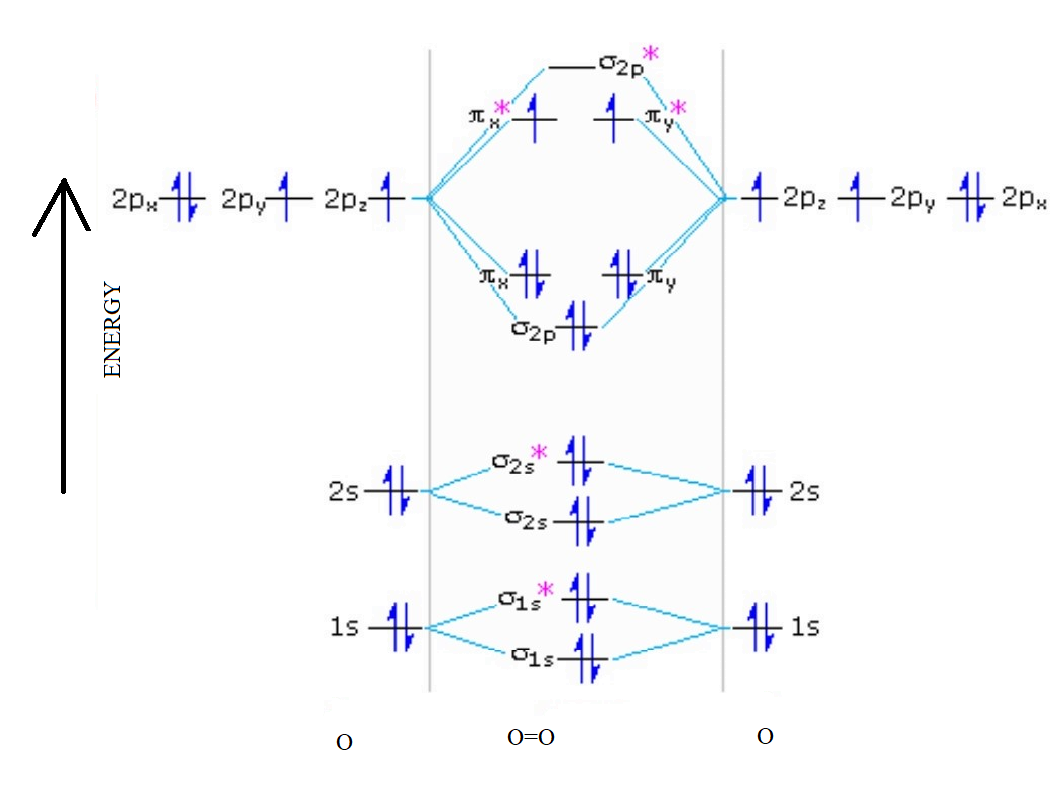
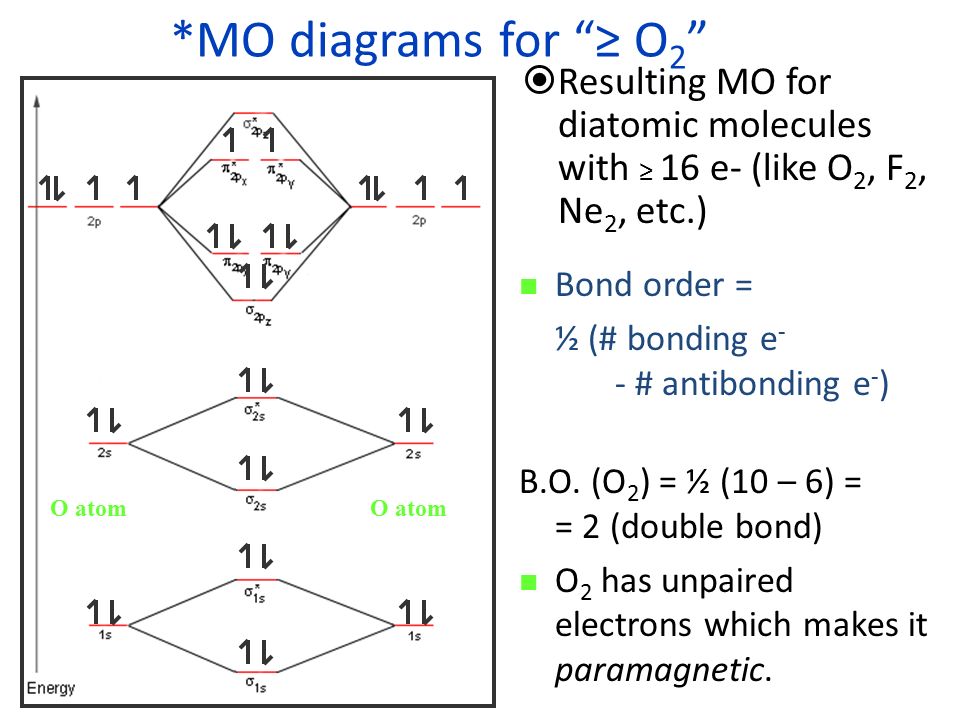
38 use the following mo diagram to find the bond order for o2.
The bond order tells us the average number of bonds between the bonded atoms. In a diatomic molecule such as `O_2`, the bond order simply tells the number of bonds between the two atoms. The bond order can be interpreted from MO diagrams using the following formula: `"Bond Order" = 1/2 [("Bonding "e^-)-("Antibonding " e^-)]` A polyatomic ion is composed of multiple covalently bonded atoms. CO₃²⁻ is a polyatomic ion composed of a carbon atom and three oxygen atoms. Predict the chemical formula for the ionic compound formed by Au³⁺ and HSO₃⁻. Au (HSO3)3. Predict the chemical formula for the ionic compound formed by NH₄⁺ and PO₄³⁻. (NH4)3PO4.
Bond order is a simple calculation, based on the number of bonding versus antibonding electrons ... The following figure illustrates our sigma and sigma-star molecular orbitals pictorially and ... There would be four electrons to fill into our molecular orbital diagram and that would force us to fill in the bonding sigma MO and the anti-bonding ...

Use the following mo diagram to find the bond order for o2.
15 Aug 2020 — Information from the MO diagram justify O2's stability and show that it's bonding order is 2. The LUMO (lowest unoccupied molecular orbital) ... Problem Details. Use the molecular orbital diagram shown below to determine which of the following diatomic species has the highest bond order. a. F 22⁻ b. F 22⁺ c. O 22⁺ d. F 2 e. Ne 22⁺. Learn this topic by watching MO Theory: Bond Order Concept Videos. All Chemistry Practice Problems MO Theory: Bond Order Practice Problems. Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the energetically-favored configuration.
Use the following mo diagram to find the bond order for o2.. Electron Configurations and Bond Orders Just as with atoms, we can write a molecular electron configuration for O2 σ2σ*2σ2π4π*2 We can also calculate the O–O bond order: BO 1 2 # bonding e # anti-bonding e 1 2 8 4 2 LCAO MO theory also predicts (correctly) that O2has two unpaired electrons. greater bond polarity in BeH2. 5.16 BeF2 uses s and p orbitals on all three atoms, and is isoelectronic with CO2. The energy level diagram for CO2 in Figure ...29 pages The bond order varies from one molecule to another. Oxygen is a diatomic molecule. Let us first know what is meant by bond order. Bond order. The bond order may be defined as half the difference between the number of electrons in bonding molecular orbitals (Nonbonding) and the number of electrons in the antibonding molecular orbital. Formula ... Molecular Orbital (MO) Theory helps us to explain and understand certain Part B - Molecular Orbital Energy Diagrams & Bond Order . + and Be2.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic ...
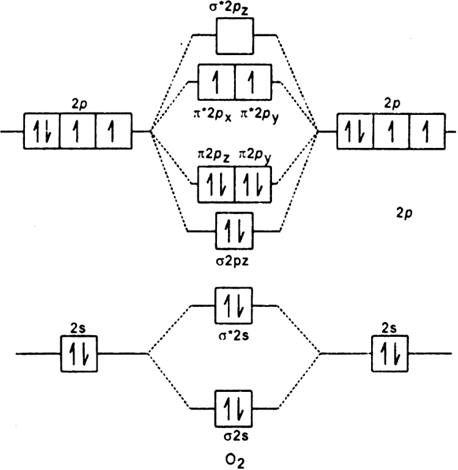
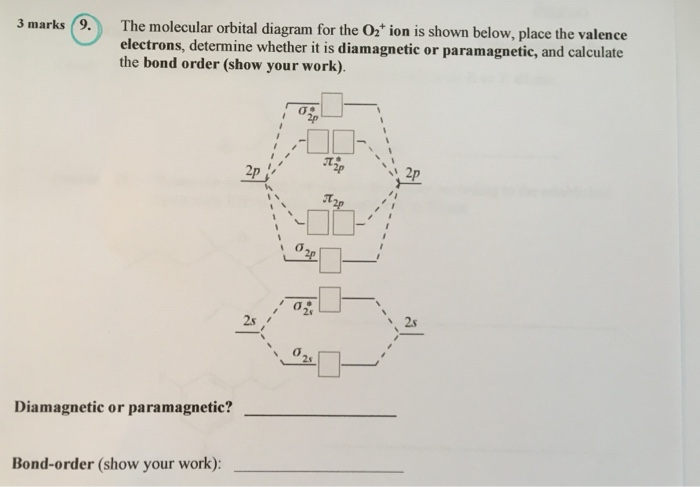
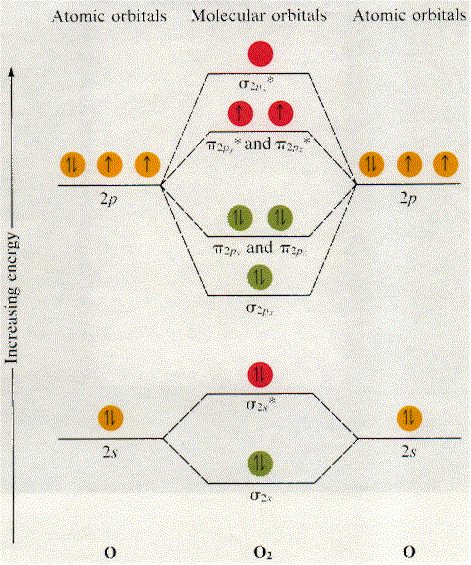
On the basis of this principle discuss the conditions for obtaining the maximum yield of SO3 in the following reaction. 2SO2(g)+ O2(g)⇌2SO3(g); ∆𝐻= - 42k.cal.(ii) Calculate the pH value of 0.01M CH3 COOH if it is 5% dissociated. Electronic structure of oxygen atom is Leaving out the 4 electrons in the 1s orbitals of two oxygen atoms constituting the molecule (represented as KK), the molecular orbital energy diagram for remaining 12 electrons of oxygen as molecule is shown: (i) Electronic configuration: (ii) Bond order: Here N b = 8; N a = 4 The two oxygen atoms in a molecule of oxygen are united through two covalent ... On the basis of this principle discuss the conditions for obtaining the maximum yield of SO3 in the following reaction. 2SO2(g)+ O2(g)⇌2SO3(g); ∆𝐻= - 42k.cal.(ii) Calculate the pH value of 0.01M CH3 COOH if it is 5% dissociated. The following is the diagram for the neutral oxygen. The bond order is ... To find the bond order of O2+ we can use the concept of Molecular Orbital Theory.5 answers · 31 votes: Hello! I actually just covered this question in my gen chem class this week. I have attached ...
Bond order of B2 molecule. So the bond order of B2 is equal to 1, which you can get by drawing the molecular orbital diagram and performing the equation Bond Order = .5 * (# of bonding electrons - # of antibonding electrons). However, when you draw the Lewis structure of B2, you get a triple bond. 4:15Remember: When two oxygen atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than ...1 Aug 2020 · Uploaded by chemistNATE O 2 2-. is 1. So, the correct order of bond order is O 2 2- O 2 − O 2 O 2 +. So, the correct answer is "Option B". Note: You should notice that bond order is indirectly proportional to the length of the bond. The higher the bond order, the shorter and stronger will be the bond. The addition of each electron in the antibonding molecular ... Bond order is also an index of bond strength, and it is used extensively in valence bond theory. Dihydrogen (H 2) This MO diagram depicts the molecule H 2, with the contributing AOs on the outside sandwiching the MO. The bonding level (lower level) is completely occupied. A bond order of one is obtained by employing the formula above ...

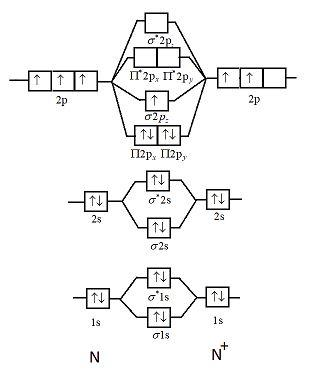
With The Help Of Molecular Orbital Theory Draw The Molecular Orbital Energy Level Diagram For N 2 Molecule Also Calculate The Bond Order And Predict The Magnetic Behaviour
2:55It is sigma2s(2)sigma2s*(2)sigma2p(2)pi2p(4)pi2p*(4)Bond order 1. It is stable. In fact, it's the perioxide ion.1 Jul 2017 · Uploaded by chemistNATE
7:11For calculation of bond order we use the formula Bond order = no of electrons in bonding - no of electrons ...15 Jun 2020 · Uploaded by Edmerls
As it can be seen from the MOT of O 2 , The electrons in the highest occupied molecular orbital are unpaired therefore it is paramagnetic in nature. Also, the bond order can be calculated as [N b − N a ] / 2 = [1 0 − 6] / 2 = 2. Therefore there is a double bond present as O = O.
4:59O2(-1) is just like regular molecular oxygen, but the extra electron causes its bond to be unstable. Its bond ...31 Jul 2020 · Uploaded by chemistNATE
Explanation: In a molecule, there are total 16 electrons. The molecular orbital configuration of molecule is as follows.. The formula for bond order is as follows. Bond order = There are 10 bonding and 6 non-bonding electrons in the orbitals according to the molecular orbital configuration.
2) Stability of molecules in terms of bond order. Bond order is defined as half of the difference between the number of electrons present in the bonding and antibonding orbitals. Bond Order = ½ ( N b – Na) The molecule is stable if N b > Na ie. bond order is positive. The molecule is unstable if N b < Na i.e. the bond order is negative or zero.
Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B. Use the drawing of the MO energy diagram to predict the bond order of Li2?. Express the bond order as an integer or fraction. Part C. Which molecules are predicted to exist in the gas phase? Check all that apply
"O"_2 is well-known to be paramagnetic, and it is one of the successes of molecular orbital theory. You can see that "CO" is not (as it has zero unpaired electrons), but "NO" is (it has one unpaired electron). Well, the MO diagram for "O"_2 is: The bond order is already calculated in the diagram.
Draw The Molecular Orbital Energy Diagram For Oxygen Molecule O2 And Show That I It Has A Double Bond Ii It Has Paramagnetic Character From Chemistry Chemical Bonding And Molecular Structure Class 11 Cbse
Molecular Orbital Theory -- Homodiatomics use the molecular orbital model to fully describe the bonding in O2+, O2, O2-, and O22-. Determine which of the following statements are true and which ...
Symmetry Free Full Text Chemical Reasoning Based On An Invariance Property Bond And Lone Pair Pictures In Quantum Structural Formulas Html
The bond order in sulfur dioxide, for example, is 1.5 the average of an S-O single bond in one Lewis structure and an S=O double bond in the other. In molecular orbital theory, we calculate bond orders by assuming that two electrons in a bonding molecular orbital contribute one net bond and that two electrons in an antibonding molecular orbital ...
Problem 7.92: Use the MO diagram in Figure 7.18b to describe the bonding in O 2 +, O 2, and O 2-. Which of the three should be stable? What is the bond order of each? Which contain unpaired electrons? First, here is the image: This diagram actually applies to this problem and to the next.
Welcome to Sarthaks eConnect: A unique platform where students can interact with teachers/experts/students to get solutions to their queries. Students (upto class 10+2) preparing for All Government Exams, CBSE Board Exam, ICSE Board Exam, State Board Exam, JEE (Mains+Advance) and NEET can ask questions from any subject and get quick answers by subject teachers/ experts/mentors/students.
Without 2s-2p mixing With 2s-2p mixing CK :O r 25 20 2s AO MO AO AO MO AO A MO energy levels for O Fa. and Ne B MO energy levels for Ba Cz and Ng 0.5 This problem has been solved! See the answer See the answer See the answer done loading
Bond order = The re are 10 bond ing and 6 non-bond ing electrons in the orbitals according to the mo lecular orbital configuration. Use the following mo diagram to find the bond order for o2.
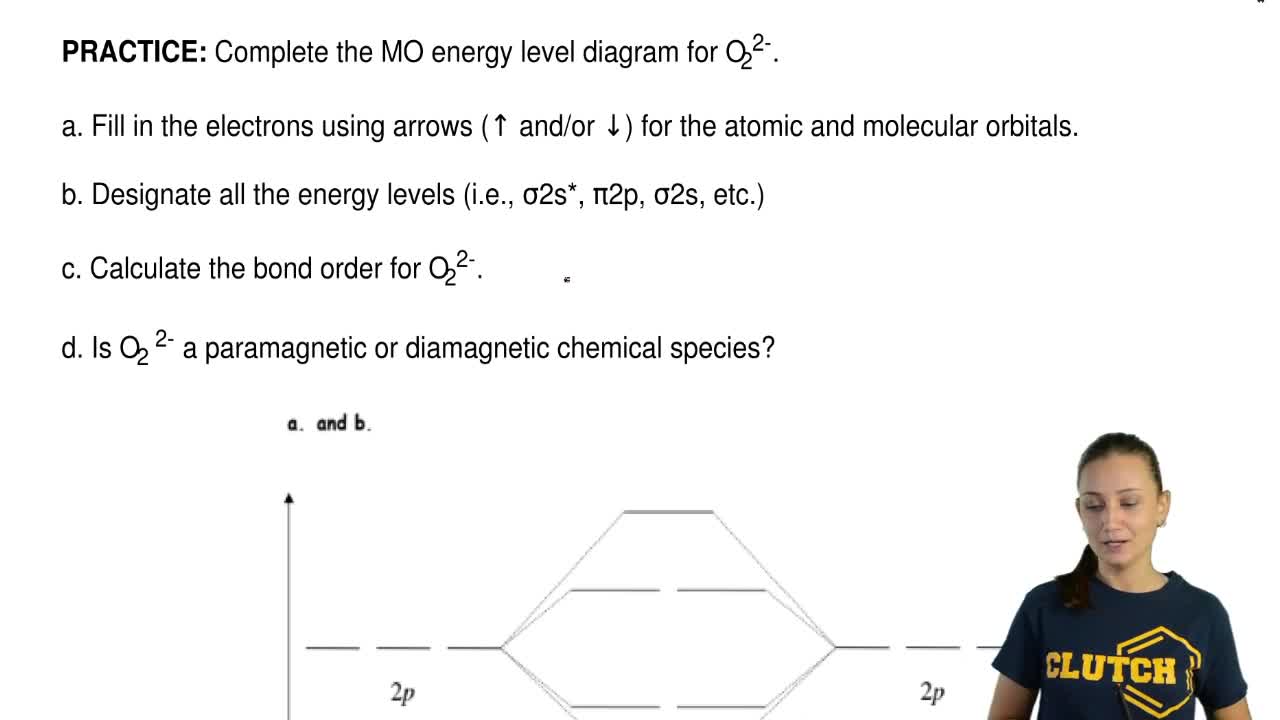
Find step-by-step Chemistry solutions and your answer to the following textbook question: Use MO diagrams and the bond orders you obtain from them to answer: (a) Is O2- stable? (b) Is O2- paramagnetic? (c) What is the outer (valence) electron configuration of O2-?.
(a) Use the MO diagram from Figure 2.18 from the textbook: The electron configuration for O 2 - is 1σ g 2 1σ u 2 2σ g 2 2π u 4 2π g 3. This leaves 1 unpaired electron and gives a bond order of 1.5. (b) Use the same MO diagram as in (a), giving an electron configuration for O 2 + of: 1σ g 2 1σ u 2 2σ g 2 2π u 4 2π g 1.
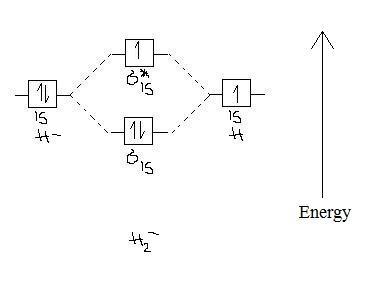
A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical ...
Answer (1 of 6): To find the bond order of O2+ we can use the concept of Molecular Orbital Theory. In this method we have to count the number of molecules in the Bonding Molecular Orbital and the Anti-Bonding Molecular Orbital. In the 1s shell there are 2 electrons in both BMO and ABMO . Also in ...
Draw The Molecular Orbital Diagram Of N2 Also Find Its Bond Order And Magnetic Character Chemistry Topperlearning Com 4s4p942zz
4a1 is the σ* 2pz antibonding MO. To obtain the bond order, look at the molecular orbitals formed and decide whether they are bonding or antibonding. BO = 1 2 (bonding e− − antibonding e−) = 1 2 [(2 + 2 + 2 + 2) − (2 + 1)] = 2.5. And this should make sense because NO+ is isoelectronic with CO, which has a bond order of 3.

Mo Diagram Of O2 O2 O2 Their Bond Order And Magnetic Char Chemistry Chemical Bonding And Molecular Structure 2562266 Meritnation Com
Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the energetically-favored configuration.
Problem Details. Use the molecular orbital diagram shown below to determine which of the following diatomic species has the highest bond order. a. F 22⁻ b. F 22⁺ c. O 22⁺ d. F 2 e. Ne 22⁺. Learn this topic by watching MO Theory: Bond Order Concept Videos. All Chemistry Practice Problems MO Theory: Bond Order Practice Problems.
15 Aug 2020 — Information from the MO diagram justify O2's stability and show that it's bonding order is 2. The LUMO (lowest unoccupied molecular orbital) ...

Draw The Molecular Orbital Diagram Of O2 And Calculate The Bond Order Is O2 Diamagnetic Or Paramagnetic Explain Your Answer Study Com

Molecular Orbital Theory Homodiatomics Use The Molecular Orbital Model To Fully Describe The Bonding In O2 O2 O2 And O22 Determine Which Of The Following Statements Are True And Which Are
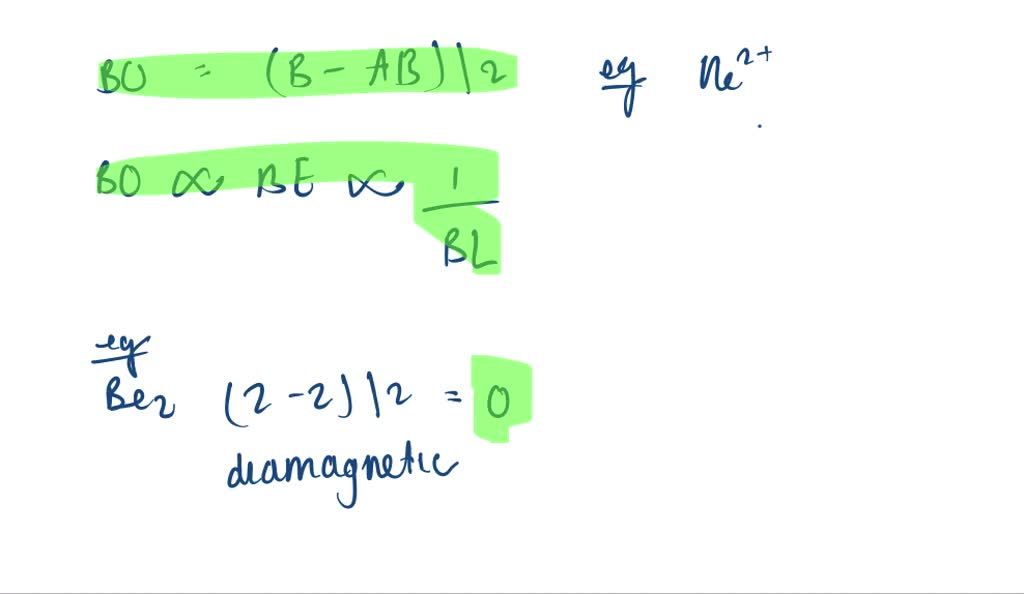
Solved 1 A Draw The Molecular Orbital Diagrams For O2 And N22 B Calculate The Bond Order For Each Diagram C Determine The Magnetic Properties For Each Diagram Para Or Diamagnetic D How














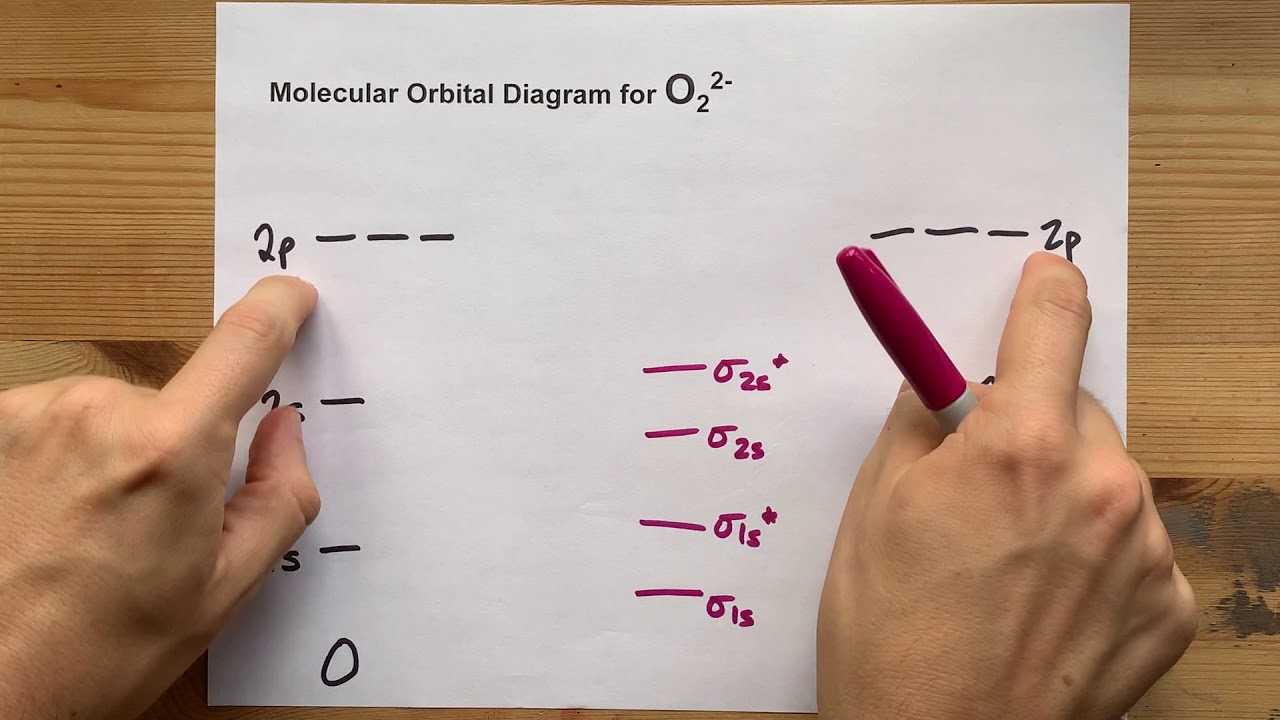


0 Response to "38 use the following mo diagram to find the bond order for o2."
Post a Comment