38 molecular orbital diagram for h2
Answer: According to the molecular orbital theory, a molecular is viable of its bond order is more than or equal to one. The bond order is defined as number of bond between to two atoms of that molecule. It is calculated as the difference of electrons in bonding molecules and anti-bonding molecul... In molecular orbital theory bond order is defined as half of the difference between the number of bonding and antibonding electrons. The bond order of diatomic nitrogen is three and it is a diamagnetic molecule. Construct the molecular orbital diagram for h2 and then identify the bond order. According to mot number of atomic orbitals combined ...
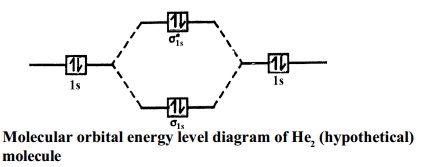
A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical ...

Molecular orbital diagram for h2
Construct the molecular orbital diagram for h2 and then identify the bond order. In fact they do. πε and jr k r mr lr defined explicitly in atkins. The orbital correlation diagram in predicts the same thing two electrons fill a single bonding molecular orbital. The procedure can be introduced by considering the h2 molecule. This video discusses how to draw the molecular orbital (MO) diagram for the H2+ ion. The bond order of H2+ is also calculated and the meaning of this number ... Construct the molecular orbital diagram for h2 and then identify the bond order. Consult a diagram of electron orbital shells. A triple covalent bond three. Molecular orbital mo theory of the h2 molecule. In molecular orbital theory bond order is defined as half of the difference between the number of bonding and antibonding electrons.
Molecular orbital diagram for h2. It is common to omit the core electrons from molecular orbital diagrams and configurations and include only the valence electrons. Figure 8. This is the molecular orbital diagram for the homonuclear diatomic Be 2 +, showing the molecular orbitals of the valence shell only. The molecular orbitals are filled in the same manner as atomic orbitals ... A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. According to the molecular orbital theory, in a supposed He2 molecule, both the if we draw its MOT DIAGRAM, 2 e's enter the Bonding molecular Orbital and 2 . The MO for $\ce{H2}$, which is shown in the figure below is taken from Wikipedia. The right side of the diagram you showed neither represents a hydrogen molecule, nor two independent (and hence equivalent) hydrogen atoms. ... How to explain the structure of HNO3 with a Molecular Orbital diagram? 7. Molecular Orbital Diagrams simplified. Megan Lim. Oct 26, 2016 · 3 min read. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding ...
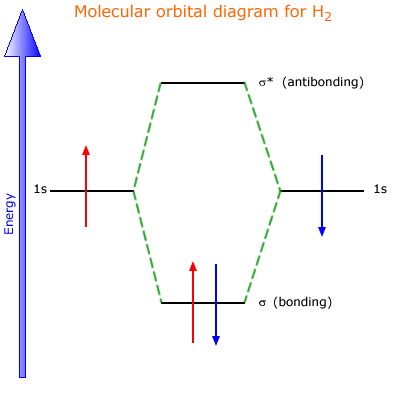
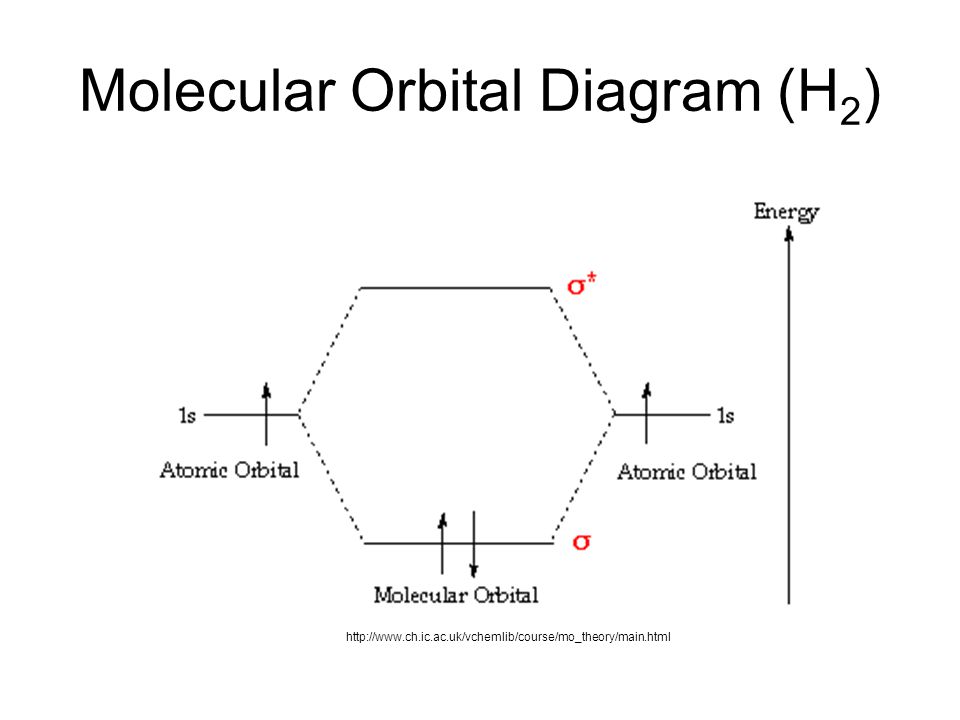
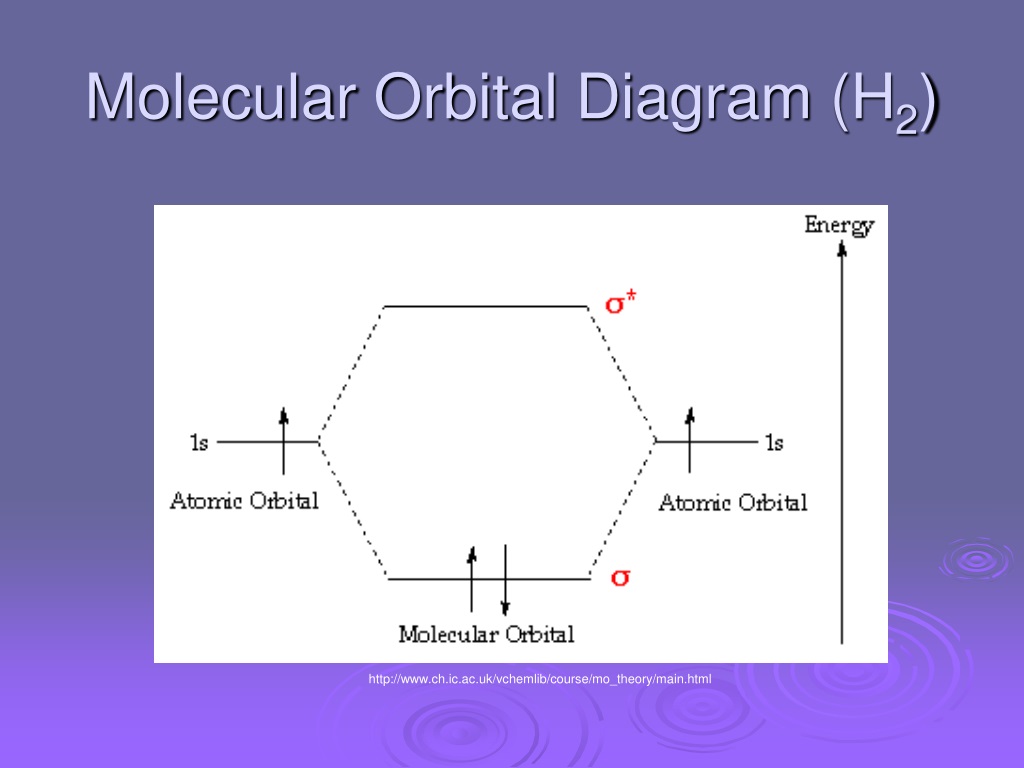
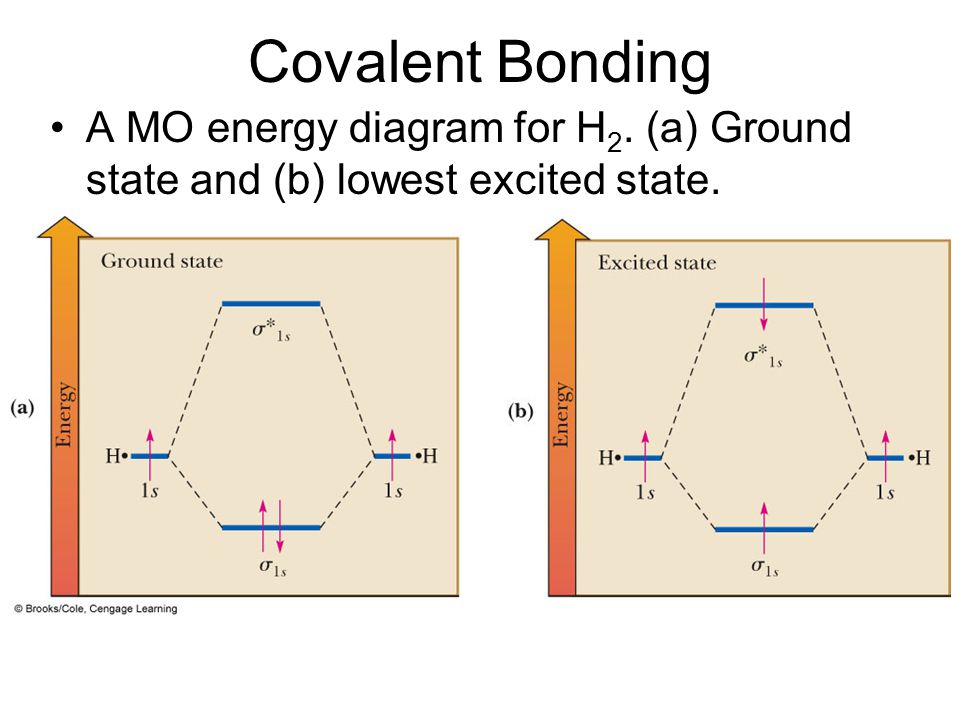
The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H 2 molecule is shown in Figure 13. On either side of the central ladder are shown the energies of the 1 s orbitals of atoms A and B, and the central two-rung ladder shows the energies of the bonding and antibonding ... Molecular orbital diagram of h2. When creating the molecular orbitals from the p orbitals notice the three atomic orbitals split into three molecular orbitals a singly degenerate σ and a doubly degenerate π orbital. Molecular orbitals of h2 and he2. Bonding mos antibonding mos and bond order. Evaluate the ground state electronic energy based ... MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine Mo · Construct The Molecular Orbital Diagram For H2 And Then Identify The Bond Order. chemical bonding molecular orbitals of h2 and he2 as before the greater the number of these nodal planes the more the electrons that occupy the orbitals are excluded from the region between the nuclei and hence the higher the energy the resulting molecular ...
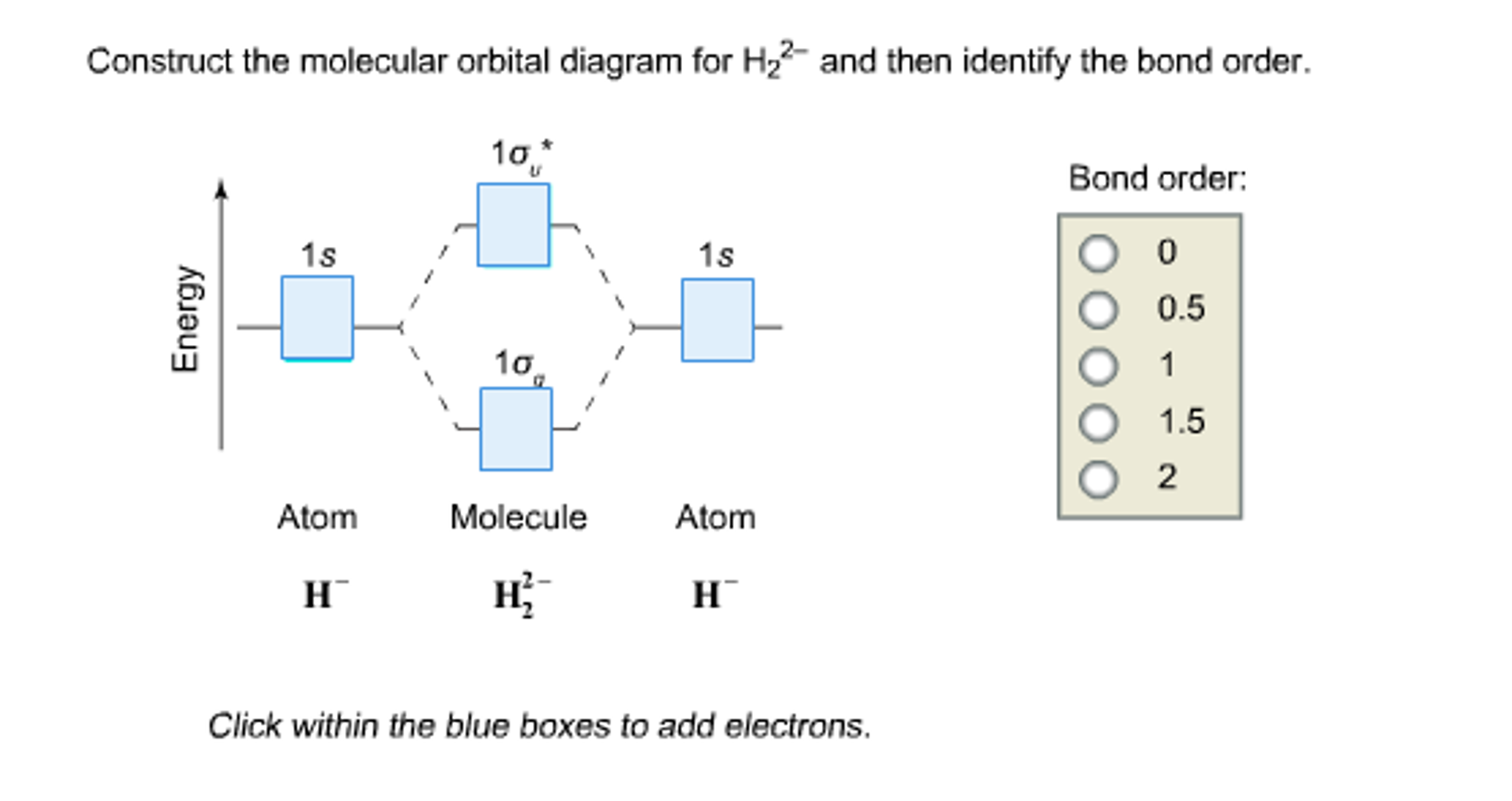
Electrons are added to molecular orbitals, one at a time, starting with the lowest energy molecular orbital. The two electrons associated with a pair of hydrogen atoms are placed in the lowest energy, or bonding, molecular orbital, as shown in the figure below. This diagram suggests that the energy of an H 2 molecule is lower than that of a ... Molecular Orbital Diagram for Hydrogen Gas (H2).Fill from the bottom up, with 2 electrons total.Bonding Order is 1, and it is Diamagnetic.sigma2s(2)Check me ... D.Construct the molecular orbital diagram for H2^+ and then identify the bond order. *need before 7:00; Question: A.Construct the molecular orbital diagram for H2^2+ and then identify the bond order. B.Construct the molecular orbital diagram for H2 and then identify the bond order. For H 2 , Bond order = 1/2 (2 - 0)=1. The bond order of H2 is 1 which shows that the hydrogen has only one bond. The BMO contains 2 electrons with opposite spins and ABMO is empty. if the number of electrons in bonding orbitals is greater than the antibonding orbital then the molecule is stable. Thus, H2 is a stable molecule.
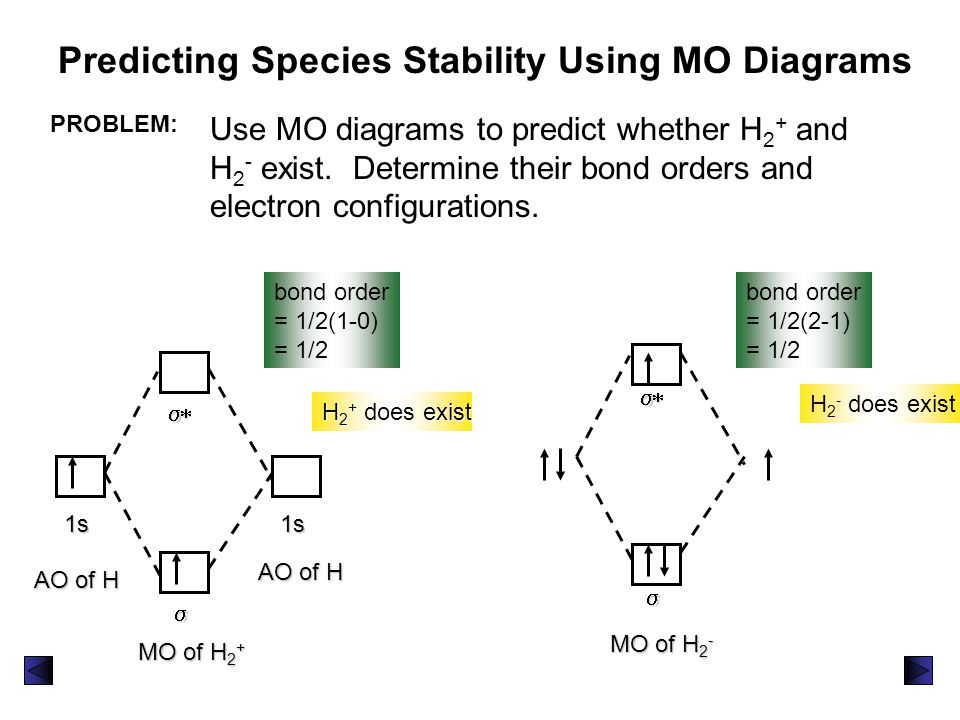
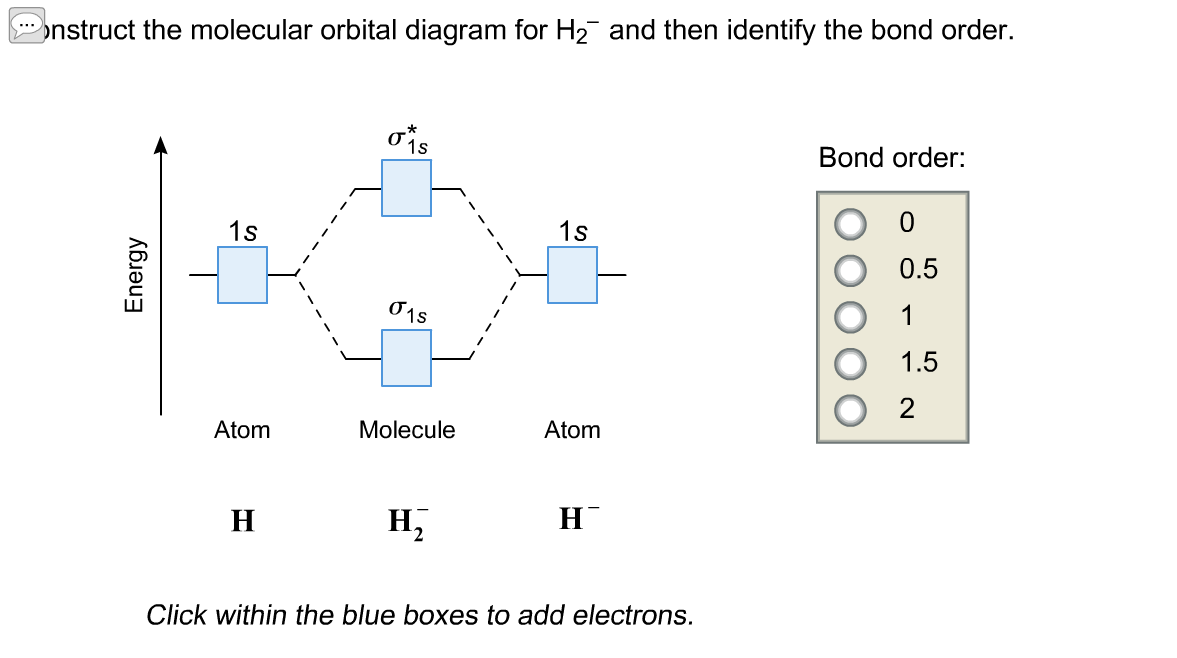
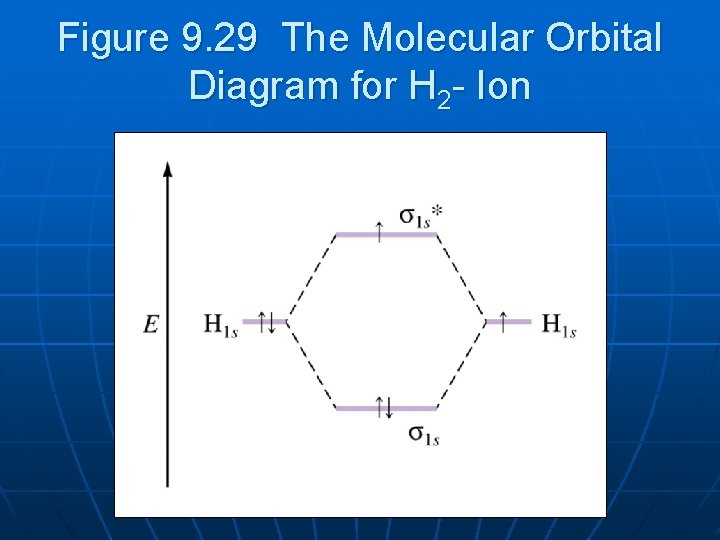
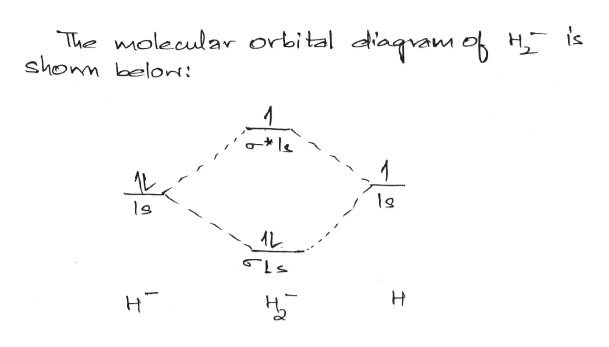
Answer (1 of 2): In order to predict the bond order, molecular orbital diagram for H2- is to be drawn. According to MOT number of atomic orbitals combined is equal to total number of molecular orbitals formed.Electronic configuration of H is 1s1. when two hydrogen atoms come closer, then on combi...
Molecular orbitals of h2 and he2. The molecular orbital energy level diagram for the h2 ion. Suppose that the ion is excited by light so that an electron moves from a lower energy to a higher energy molecular orbital. πε and jr k r mr lr defined explicitly in atkins. Sketch the molecular orbitals of the h2 ion and draw its energy level diagram.
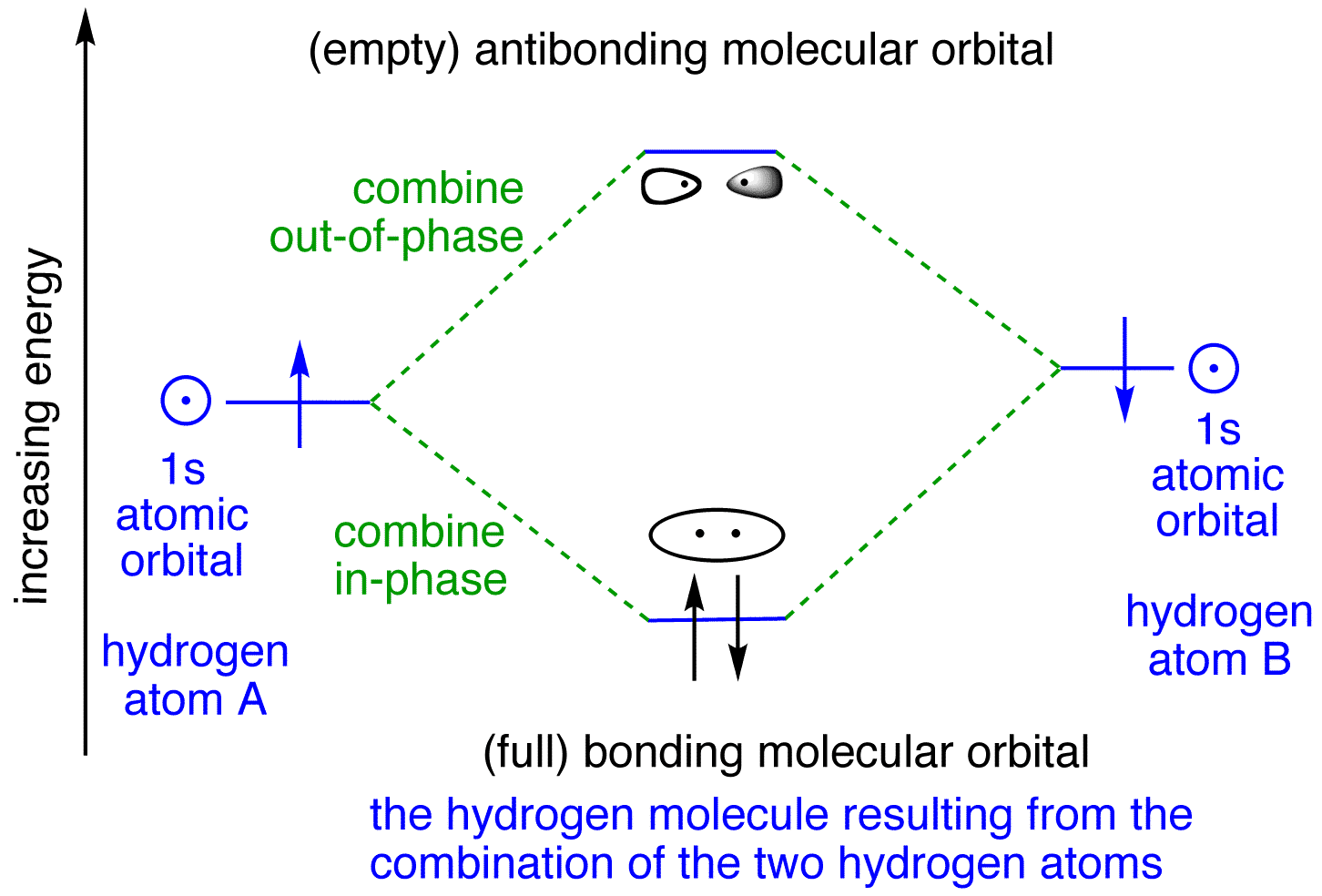
LCAO MO Energy Diagram for H2 Energy H-H ∆E1 ∆E2 • ∆E2> ∆E1, so the antibonding orbital is always more anti-bonding than the bonding orbital is bonding H2molecule: two 1s atomic orbitals combine to make one bonding and one antibonding molecular orbital. Ha Hb
Energy level diagram for Molecular orbitals. ... If N b = Na,the molecule is again unstable because influence of electrons in the antibonding molecular orbital is greater than the bond influence of electron in the bonding molecular orbitals. 2) Stability of molecules in terms of bond order.
Molecular Orbital (MO) Theory of the H2 molecule: Following the MO treatment of H2+, assume the (normalized) ground electronic state wavefunction is given by: gs ψψ αβ ... Qualitative MO theory orbital diagram for homonuclear diatomics composed of 1st or 2nd row elements:
1)H2+. Molecular orbital energy level for H2+. The electronic configuration of H2+. Answer to Create an MO diagram for H2+ H2 and H Post the Lumo, lumo -, homo, homo + near its energy level. Molecular Orbital (MO) Theory of the H2 molecule: Following the MO treatment of H2+, assume the (normalized) ground electronic state wavefunction is .
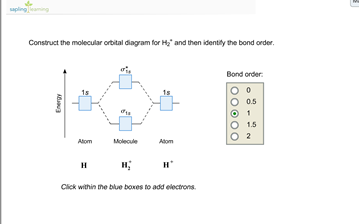
Science. Chemistry. Chemistry questions and answers. Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes to add electrons. Question: Construct the molecular orbital diagram for H2- and then identify the bond order.
Answer to Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes. The hydrogen atom is the simplest atom, and its molecule \ (\ce {H2}\) is get a sigma (s) bonding orbital, denoted as s1s in the diagram here. Chemical bonding - Molecular orbitals of H2 and He2: The procedure can ...
Description of the molecular orbitals of the h2 molecule with an introduction to molecular orbital diagrams. Each boron atom has one 2s and three 2p valence orbitals. In order to predict the bond order molecular orbital diagram for h2 is to be drawn. Molecular orbitals of h 2 the molecular orbital approach is one explanation for the ceh h bond.
Well, build the molecular orbital (MO) diagram. Each hydrogen atom contributes one electron, and thus, "H"_2^(-) has three electrons while "H"_2^(+) has one. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to MO theory to form one sigma_(1s) and one sigma_(1s)^"*" MO by conservation of orbitals.
H2 Molecular Orbital Diagram. MO diagram of dihydrogen. Bond breaking in MO diagram. The smallest molecule, hydrogen gas exists as dihydrogen (H-H) with a single covalent bond between two hydrogen atoms. As each hydrogen atom has a single 1s atomic orbital for its electron, the bond forms by overlap of these two atomic orbitals. In the figure ...
Construct the molecular orbital diagram for h2 and then identify the bond order. Consult a diagram of electron orbital shells. A triple covalent bond three. Molecular orbital mo theory of the h2 molecule. In molecular orbital theory bond order is defined as half of the difference between the number of bonding and antibonding electrons.
This video discusses how to draw the molecular orbital (MO) diagram for the H2+ ion. The bond order of H2+ is also calculated and the meaning of this number ...
Construct the molecular orbital diagram for h2 and then identify the bond order. In fact they do. πε and jr k r mr lr defined explicitly in atkins. The orbital correlation diagram in predicts the same thing two electrons fill a single bonding molecular orbital. The procedure can be introduced by considering the h2 molecule.
When An Electron Of H2 Is Promoted To The Excited State Does The Molecule Continue To Exist Or Does Its Bond Break Quora
Molecular Orbital Theory Chemistry Encyclopedia Structure Number Molecule Atom Bond Order Multiple Bonds

















0 Response to "38 molecular orbital diagram for h2"
Post a Comment