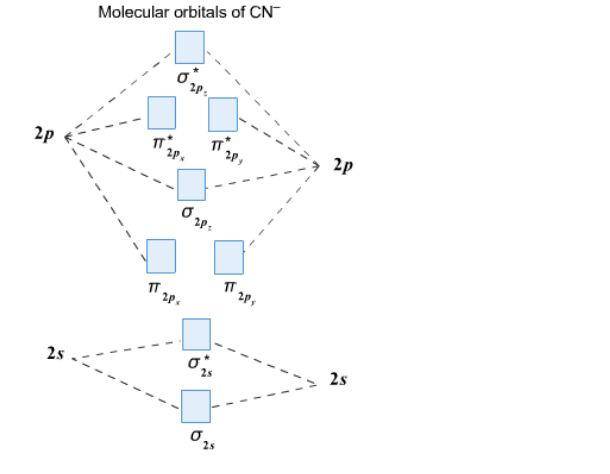
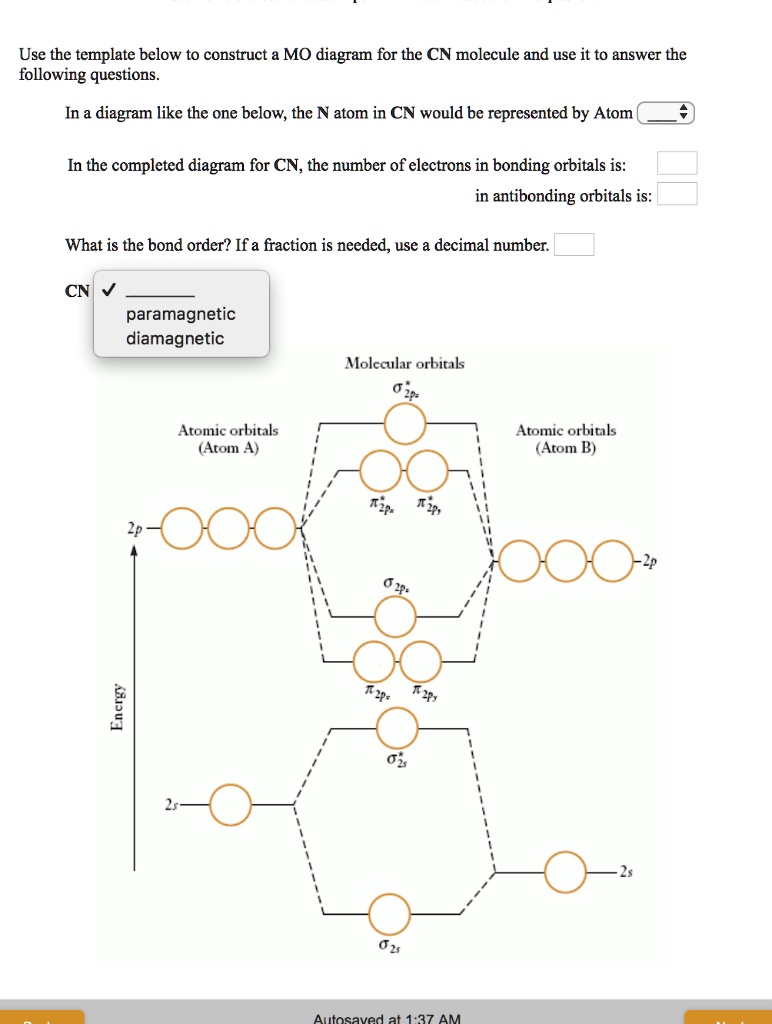
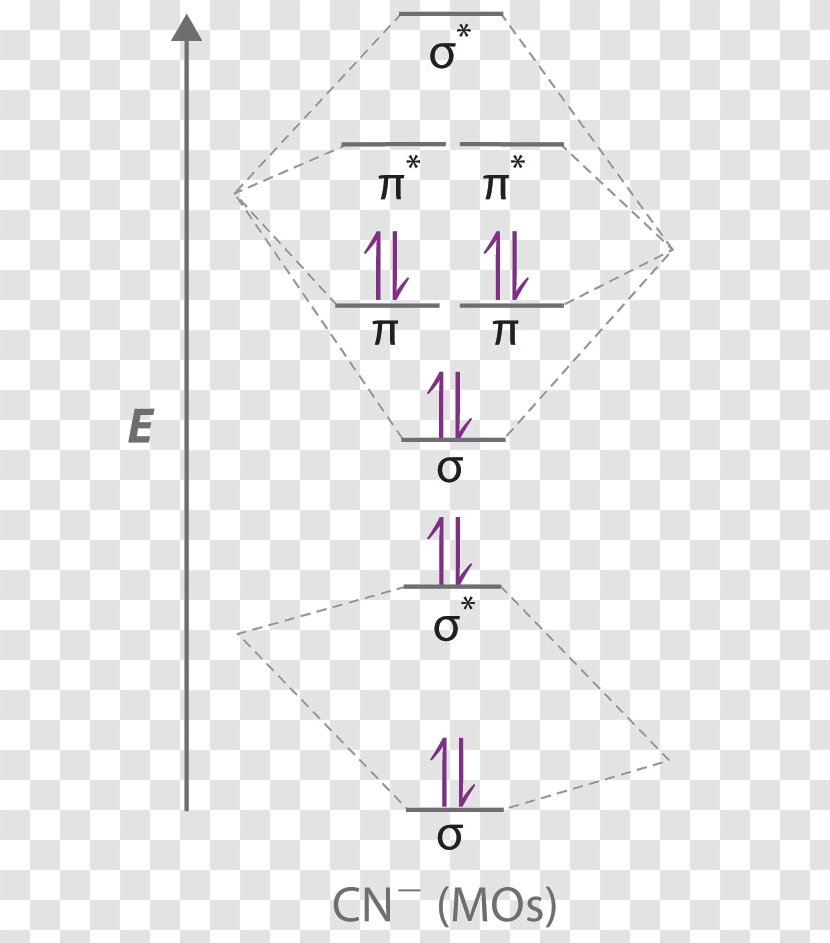
38 mo diagram for cn
Dr. Shields shows you how to draw the MO correlation diagram for cyanide (CN-), calculate the MO bond order, and write the MO electron ... When two atomic orbitals combine, two molecular orbitals are formed. One is known as bonding molecular orbital and the other is called an anti-bonding molecular ...
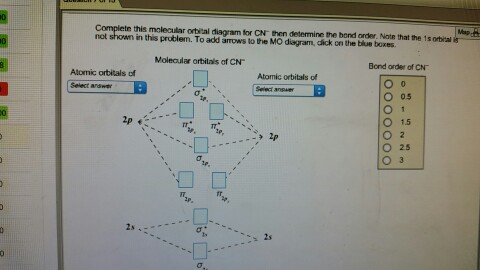
To add arrows to the MO diagram, click on the blue boxes. Labels. Chemistry. Answers. Bond order of CN- is 2 =No of antibonding ...
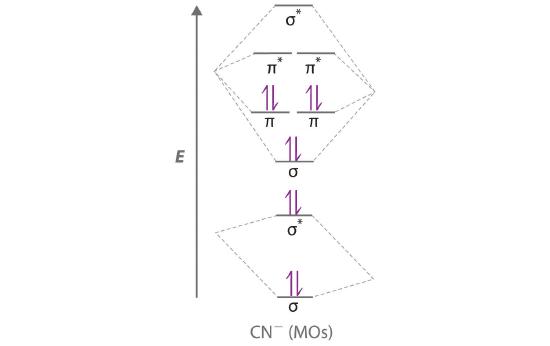
Mo diagram for cn
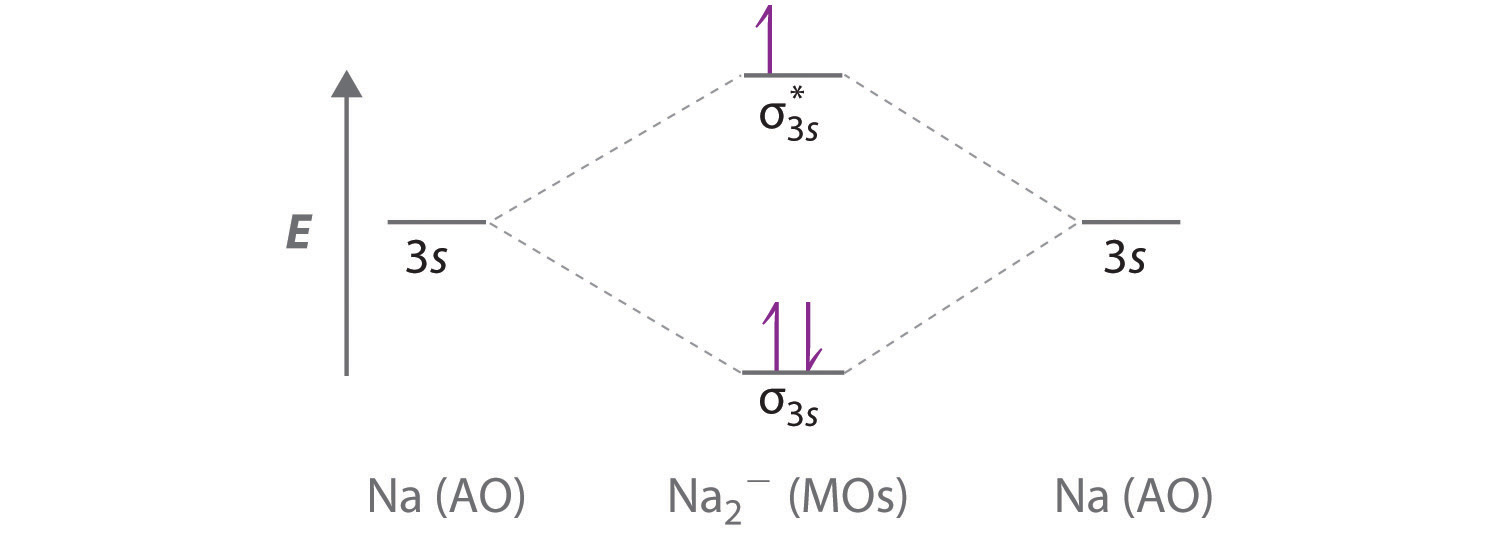
The total energy of the molecule must be stabilised. This means that very occasionally an occupied MO can move up in energy as long as the occupied MOs move ... How to make molecular Orbital diagramhttps://www.youtube.com/watch?v=UYC-ndQ6Lww&t=6s. Because each alkali metal (M) has an ns1 valence electron configuration, the M2 molecule has two valence electrons that fill the σns bonding ...
Mo diagram for cn. We're being asked to complete the molecular orbital diagram of CN- and then determine the bond order. To do so, we shall follow these steps: Step 1: Calculate ... There are no unpaired electrons in MO diagram; CN- is diamagnetic. HOMO: Highest Occupied Molecular Orbital is the highest orbital that is filled with electrons ... This video is about MO Diagram #3 - CN- ... Molecular Orbital Diagrams Heteronuclear Diatomic HF. Alicia Rae Welden. Alicia Rae Welden. CN– lewis structure contains two atoms (carbon and nitrogen) connected with a triple bond. There is two lone pair present, one on nitrogen and the other on ...
Because each alkali metal (M) has an ns1 valence electron configuration, the M2 molecule has two valence electrons that fill the σns bonding ... How to make molecular Orbital diagramhttps://www.youtube.com/watch?v=UYC-ndQ6Lww&t=6s. The total energy of the molecule must be stabilised. This means that very occasionally an occupied MO can move up in energy as long as the occupied MOs move ...

Scielo Brasil Exploring The Metallochromic Behavior Of Pentacyanidoferrates In Visual Electronic And Raman Spot Tests Exploring The Metallochromic Behavior Of Pentacyanidoferrates In Visual Electronic And Raman Spot Tests

Figure 2 From Photoredox Reactions Of Hg Cn 2 Fe Cn 6 4 And Hgco2 Cn 10 6 Induced By Inner Sphere Metal To Metal Charge Transfer Excitation Semantic Scholar
Orbital Structure 35 Images The Effects Of Orbital Overlap On The Electronic Band Molecular Orbital Diagram Cn Untpikapps Electronic Orbitals
Properties Of Vacancies And N Doping In Monolayer G Zno First Principles Calculation And Molecular Orbital Theory Analysis

How Can One Tell From The Mo Diagram Of The Cyanide Ion That The Homo Is Carbon Centred Chemistry Stack Exchange






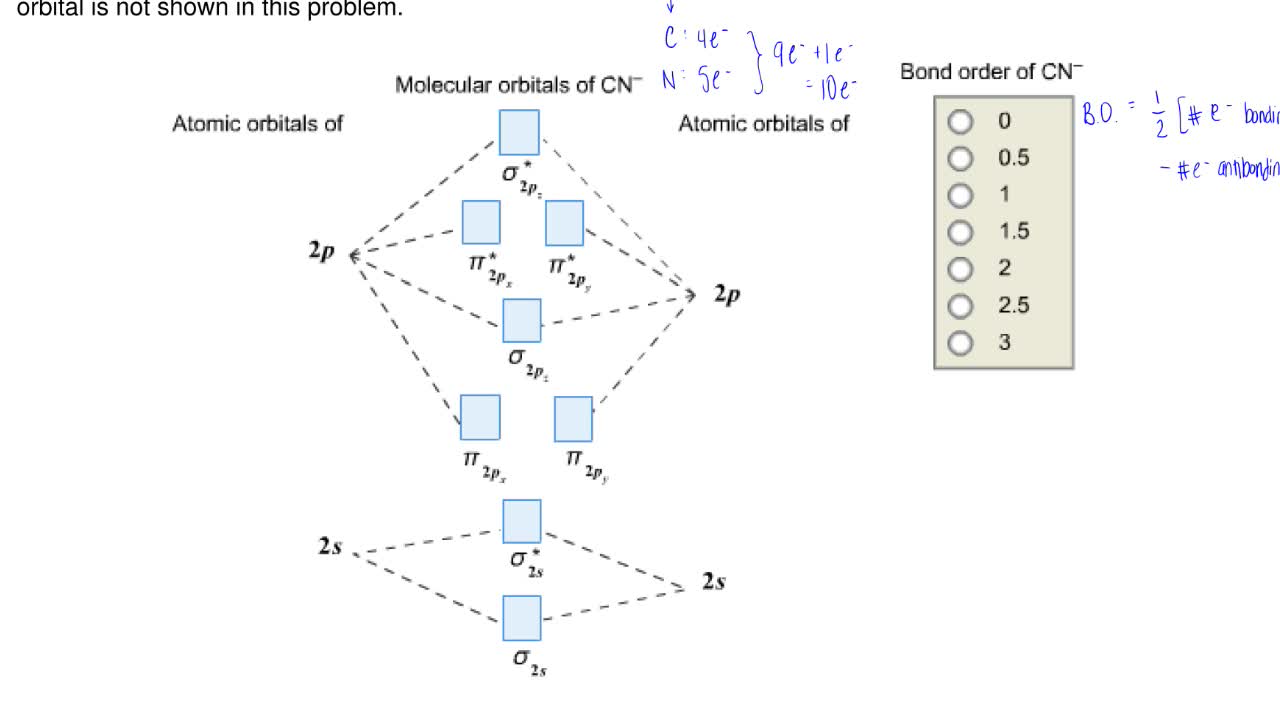







0 Response to "38 mo diagram for cn"
Post a Comment