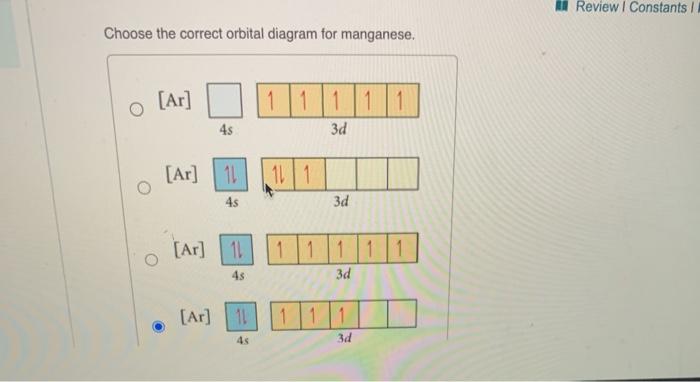
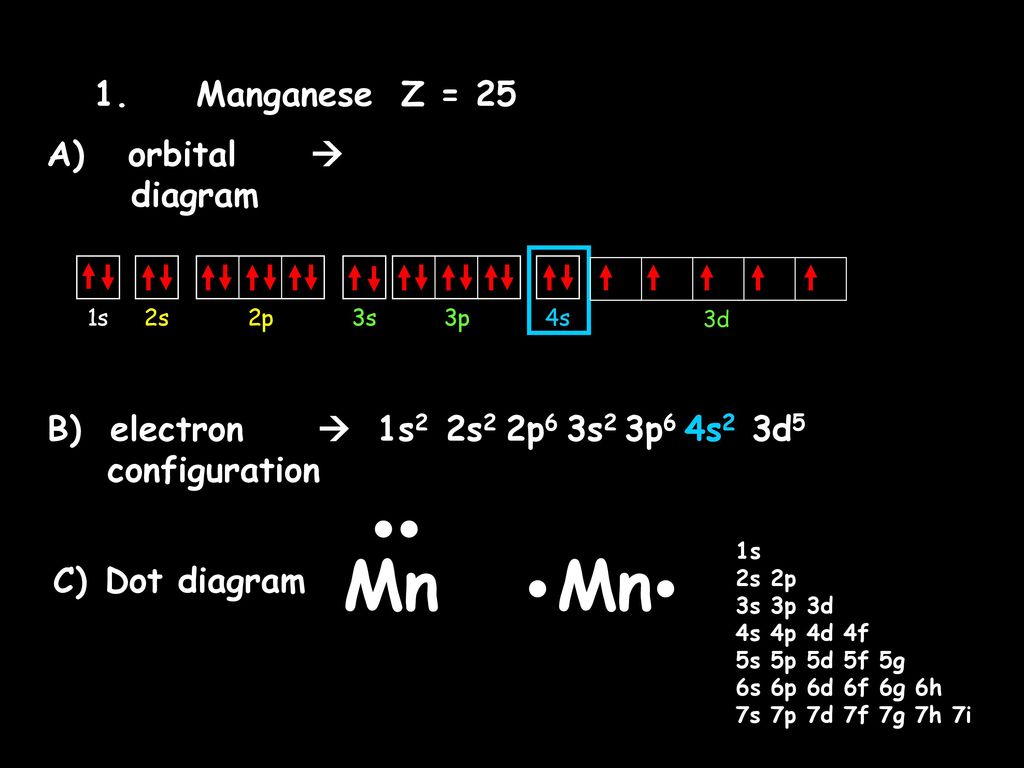
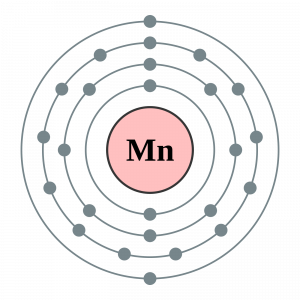
37 choose the correct orbital diagram for manganese.
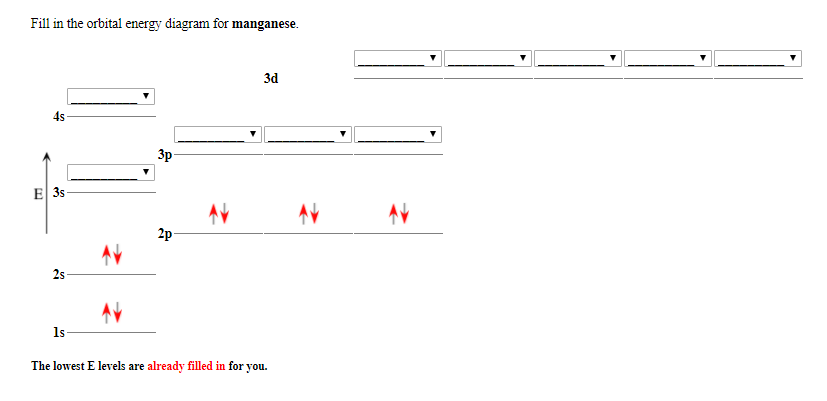
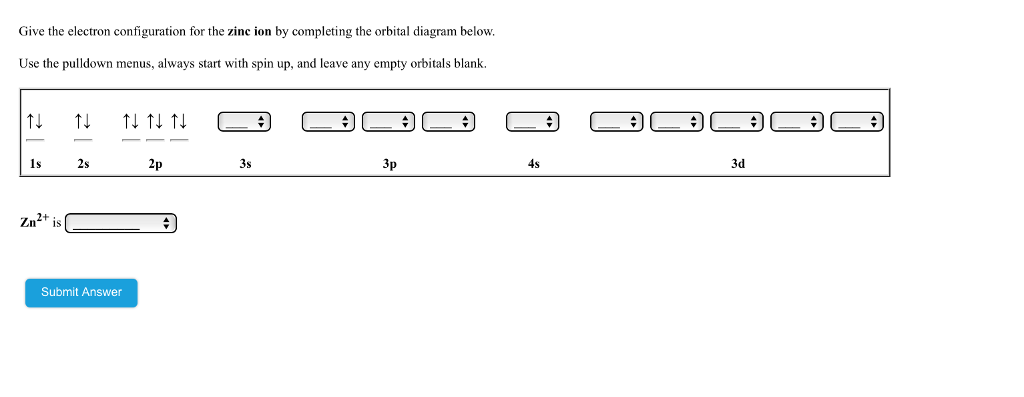
Transcribed Image Textfrom this Question. Give the electron configuration for manganese by completing the orbital diagram below. Use the pulldown menus, always start with spin up, and leave any empty orbitals blank. 4s 3d 1s 2s 2p 3s 3p Mn is Submit Answer. Choose the orbital diagram that represents the ground state of N. Give the ground state electron configuration for Se. [Ar]4s23d 104p4 [Ar]4s24dl04p4 [Ar]4s23dl04p6 [Ar]4s23dl0 [Ar]3dl04p4 Identify the clement that has a ground state electronic configuration of [Kr)5s24d5.
The orbital diagram has nine boxes with two arrows in the first seven and single arrows in the last two Write the electron configuration and draw the orbital notation for atoms of oxygen and sulfur. 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p
Choose the correct orbital diagram for manganese.
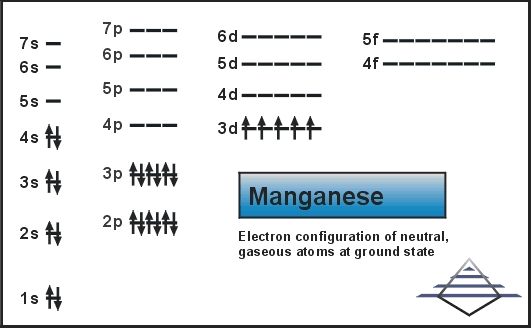
Best Answer. Copy. 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^5 is the orbital notation for manganese. Wiki User. ∙ 2015-06-01 20:20:24. This answer is: Helpful. Not Helpful. 🙏. Crystal field theory was developed by considering two compounds: manganese (II) oxide, MnO, and copper (I) chloride, CuCl. Octahedral Crystal Fields. Each Mn 2+ ion in manganese (II) oxide is surrounded by six O 2- ions arranged toward the corners of an octahedron, as shown in the figure below. MnO is therefore a model for an octahedral complex ... Orbital Diagram. 1s ... The steel in railroad tracks can contain as much as 1.2% manganese. It is crucial to the effectiveness of vitamin B1. Sources Most abundant ores are pyrolusite (MnO2), psilomelane [(Ba,H2O)2Mn5O10] and rhodochrosite (MnCO3). Pure metal produced by mixing MnO2 with powered Al and ignited in a furnace.
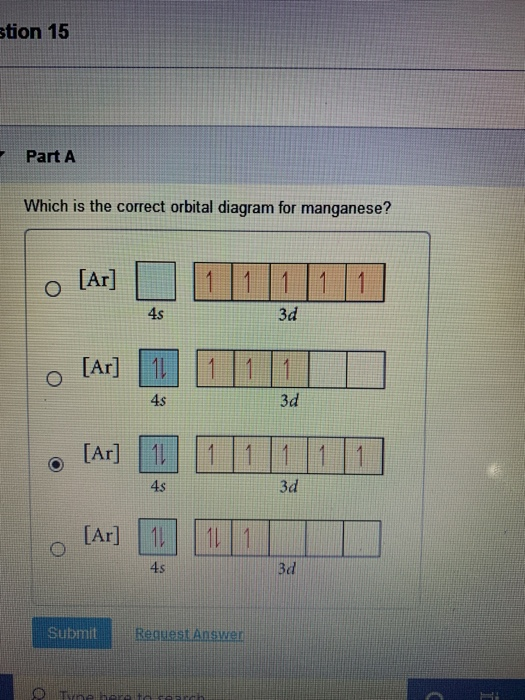
Choose the correct orbital diagram for manganese.. The electron configuration for manganese is 1s2 2s2 2p6 3s2 3p6 4s2 3d5. It can be shortened to [Ar] 4s2 3d5, where the [Ar] represents argon, the last element in the third row of the periodic table, whose electrons fill every shell prior to the 4s-orbital. The first number in each grouping represents the energy level. Refer to the explanation. The electron configuration of manganese, atomic number 25, is "1s"^2"2"^2"2p"^6"3s"^2"3p"^6"3d"^5"4s"^2". The diagram below represents the electron configuration as an orbital diagram. Correct option is . C. 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 5. The atomic number of Manganese (Mn) ... Thus answer is option C. Was this answer helpful? 0. 0. Similar questions. If each orbital can take maximum of three electrons (elements), then the number of elements in 3rd period are X: (Write answer as X/9 ) ... Diagram set > Real Life ... Show transcribed image text. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (2 ratings) Transcribed image text: stion 15 Part A Which is the correct orbital diagram for manganese? 3d Submit Request Answer MW.
Orbital Diagram. 1s ... The steel in railroad tracks can contain as much as 1.2% manganese. It is crucial to the effectiveness of vitamin B1. Sources Most abundant ores are pyrolusite (MnO2), psilomelane [(Ba,H2O)2Mn5O10] and rhodochrosite (MnCO3). Pure metal produced by mixing MnO2 with powered Al and ignited in a furnace. Crystal field theory was developed by considering two compounds: manganese (II) oxide, MnO, and copper (I) chloride, CuCl. Octahedral Crystal Fields. Each Mn 2+ ion in manganese (II) oxide is surrounded by six O 2- ions arranged toward the corners of an octahedron, as shown in the figure below. MnO is therefore a model for an octahedral complex ... Best Answer. Copy. 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^5 is the orbital notation for manganese. Wiki User. ∙ 2015-06-01 20:20:24. This answer is: Helpful. Not Helpful. 🙏.
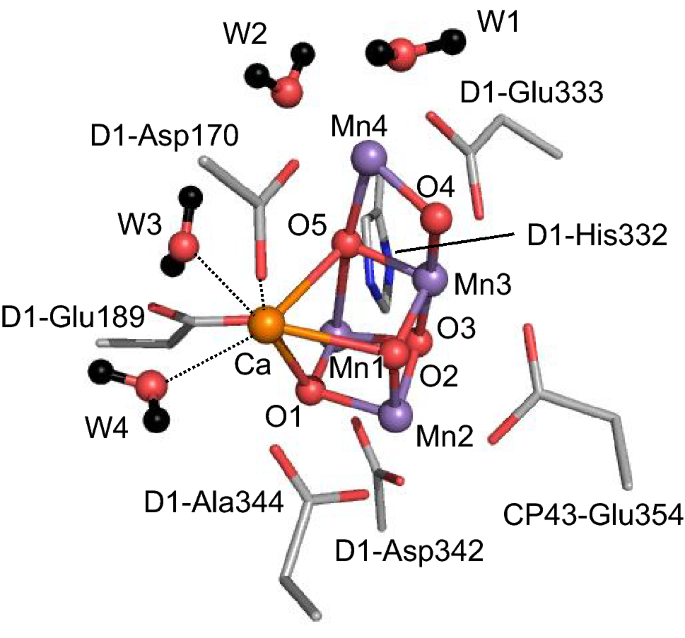
Role Of Redox Inactive Metals In Controlling The Redox Potential Of Heterometallic Manganese Oxido Clusters Springerlink
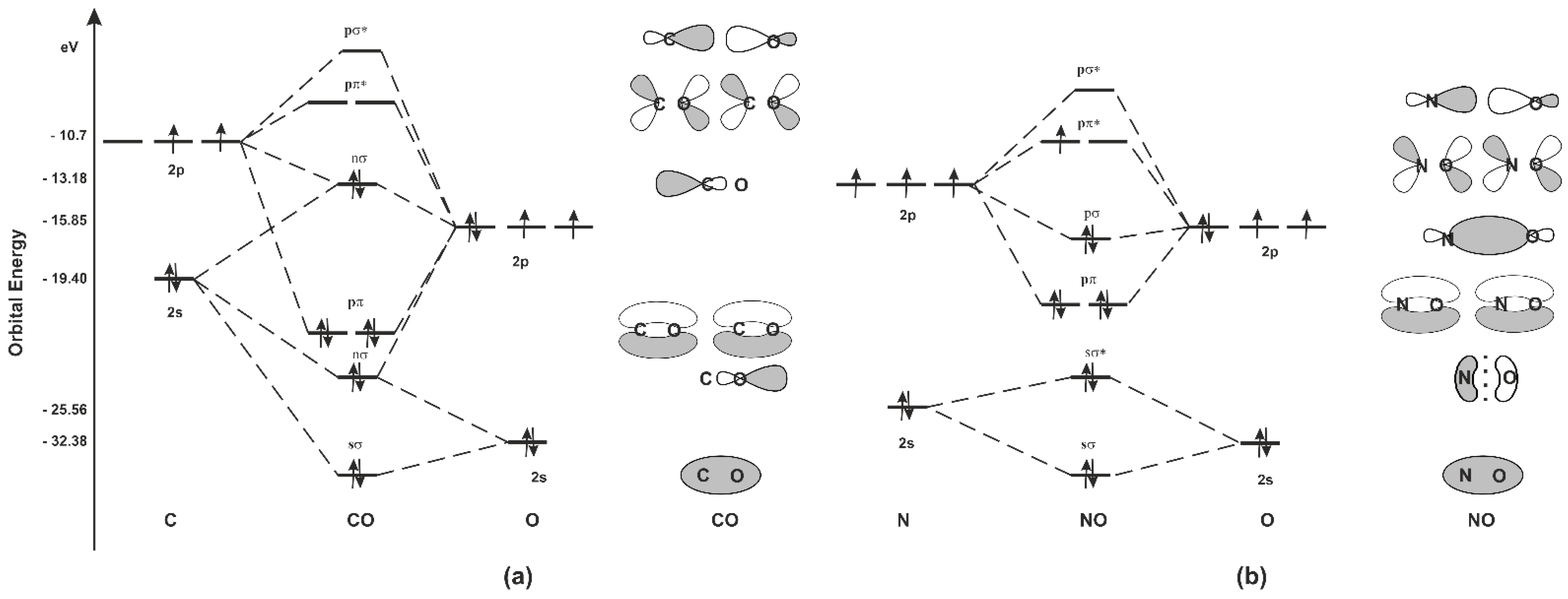
Ijms Free Full Text Carbon Monoxide And Nitric Oxide As Examples Of The Youngest Class Of Transmitters Html
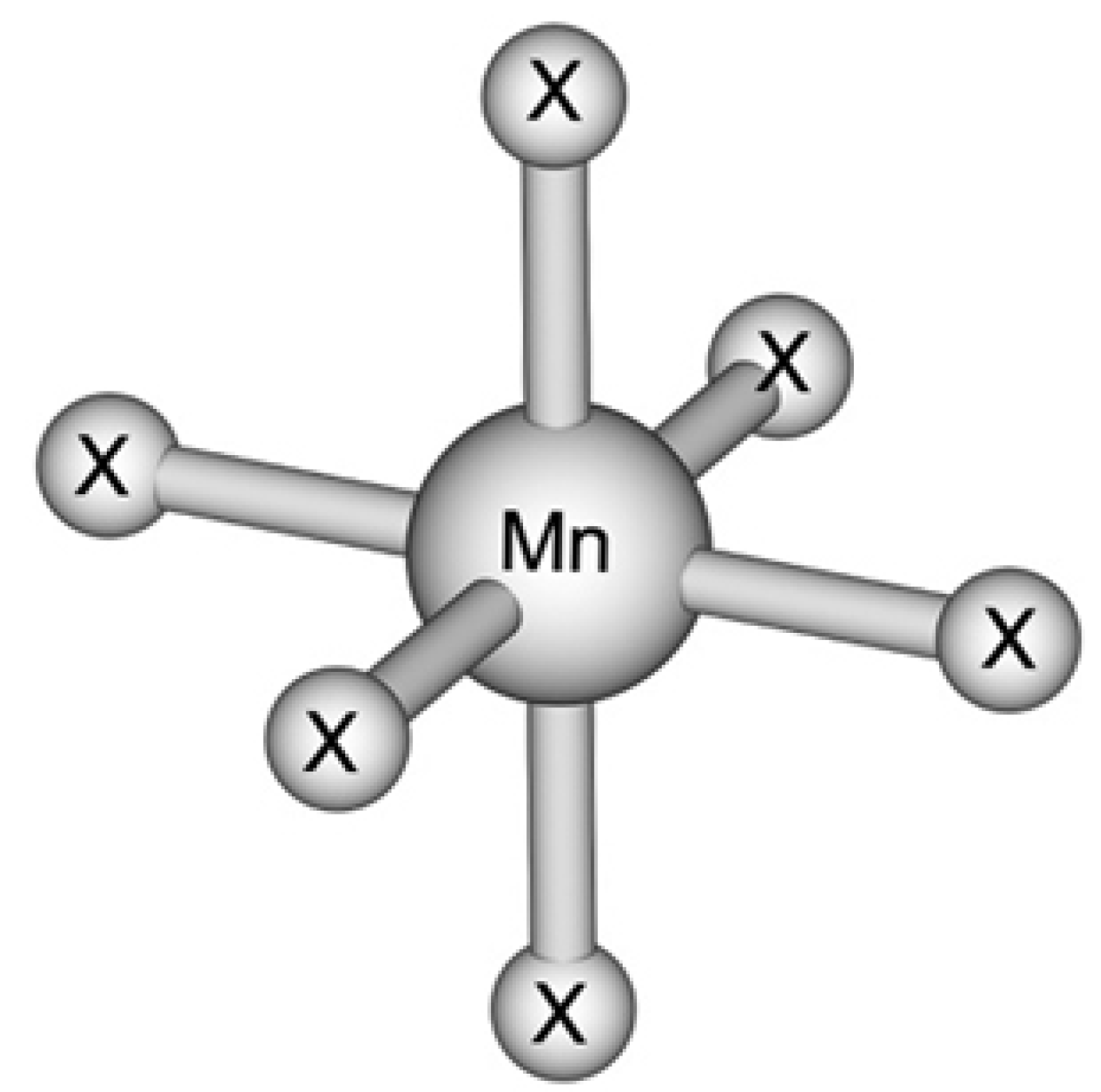
Ijms Free Full Text Modelling Of Octahedral Manganese Ii Complexes With Inorganic Ligands A Problem With Spin States Html

Effect Of Ligand Substituents And Tuning The Spin State Switching In Manganese Iii Complexes Dalton Transactions Rsc Publishing



















0 Response to "37 choose the correct orbital diagram for manganese."
Post a Comment